Acyl phosphates and thioesters are high energy carboxylic acid derivatives, a fact of profound importance in biochemistry. Acyl phosphates and thioesters are 'activated.' They can be spontaneously converted to esters, carboxylic acids and amides without the additional input of energy.
an example of the importance of these concepts in biochemistry is in the purpose of the cofactor coenzyme A (CoA). By forming a thioester with acyl groups, coenzyme A provides them with a high transfer potential. Coenzyme A is a carrier of activated acyl groups.
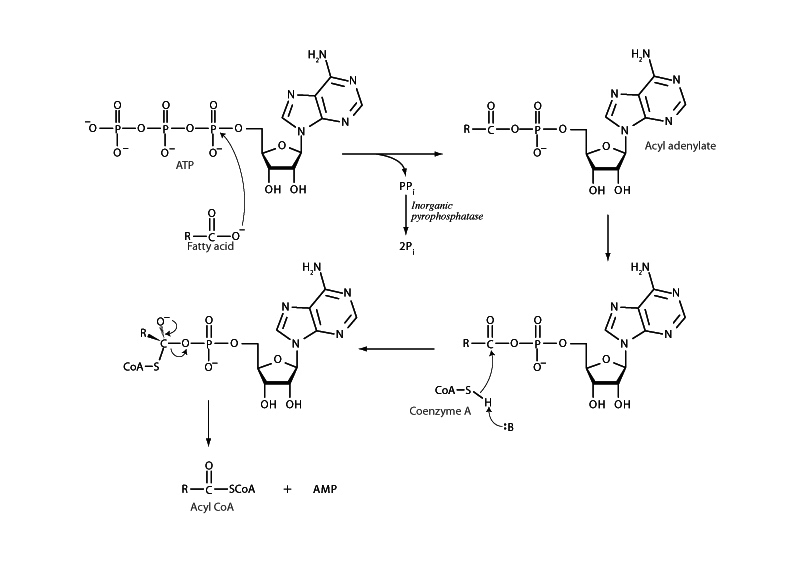
Prior to β-oxidation, fatty acids are linked to coenzyme A by acyl CoA synthetase. ATP cleavage drives the formation of acyl CoA. The enzyme uses ATP to first transform the fatty acid into an even higher energy carboxylic acid derivative than a thioester, a type of a type of phosphate ester called an acyl adenylate. Formation of acyl adenylate occurs by transfer of adenyl phosphate onto carboxylate, which breaks the bond between the alpha and beta phosphate groups of ATP and releases pyrophosphate (PPi). Subsequent cleavage of pyrophosphate by pyrophosphatase drives this reaction forward. The sulfhydral group of CoA then attacks the acyl adenylate to form a tetrahedral intermediate. Proceeding through the acyl substitution pathway, the intermediate then collapses to form acyl CoA and release AMP.