Interdisciplinary Note (2 of 16)
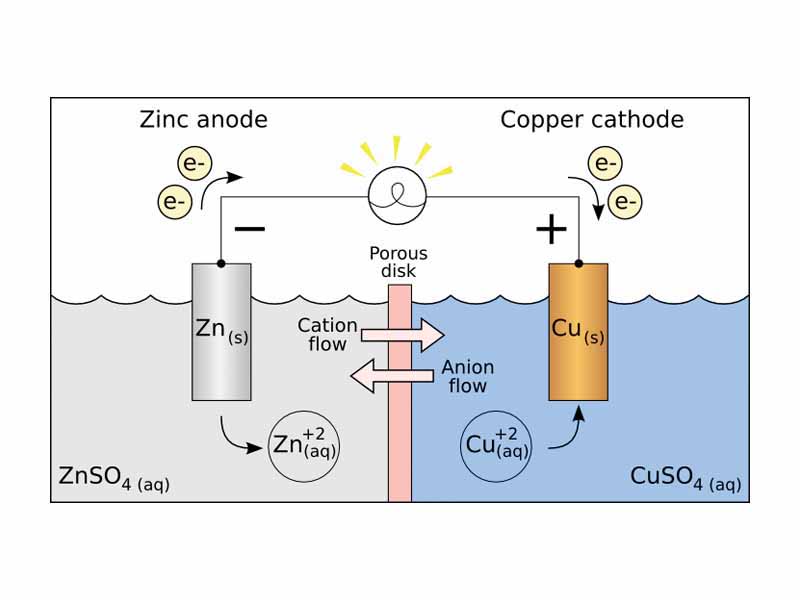
Through both our practical everyday encounters with electrical technology and the core model systems for DC current used in physics education, we take on the mental image of electrical current as electrons moving through a metallic conductor. Within a metal, the atoms are stacked within a lattice. The very outermost electrons flow like an sea. This is the image of electrical current. A potential is applied and the electrons flow. This is not wrong. That's electric current. Electric current is the flow of electric charge. However, there are many other kinds of current.
One very important type of electric current, for example, involves the movements of ions within an aqueous solution. Apply an electrical potential across a concentrated NaCl solution and the sodium ions in the solution start moving towards the negative electrode. The chloride ions start moving towards the positive electrode. The movements of both ions together are represented as positive current from the positive electrode to the negative. Those movements of ions in solution are every bit as much electric current as the movement of electrons in a wire.
To measure the conductivity of a salt solution you use a type of probe that applies a voltage between two electrode. The drop in voltage required to produce a given current in the through the electrode measures the resistance of the water. As we know, the unit of electrical resistance is the ohm. If we take the reciprocal of that - believe it or not - we have a unit called the "mho". If resistance goes down, the mhos go up. The basic unit of conductivity is the "mho/cm", otherwise known as 1 Siemen. 1 Siemen is a very, very, very high conductivity, so nobody uses it. The conductivity of seawater, for example, is measured in milimhos/cm (mS). It's approximately 55 mS, and you would express the conductivity of freshwater in micromhos/cm.