Interdisciplinary Note (5 of 9)
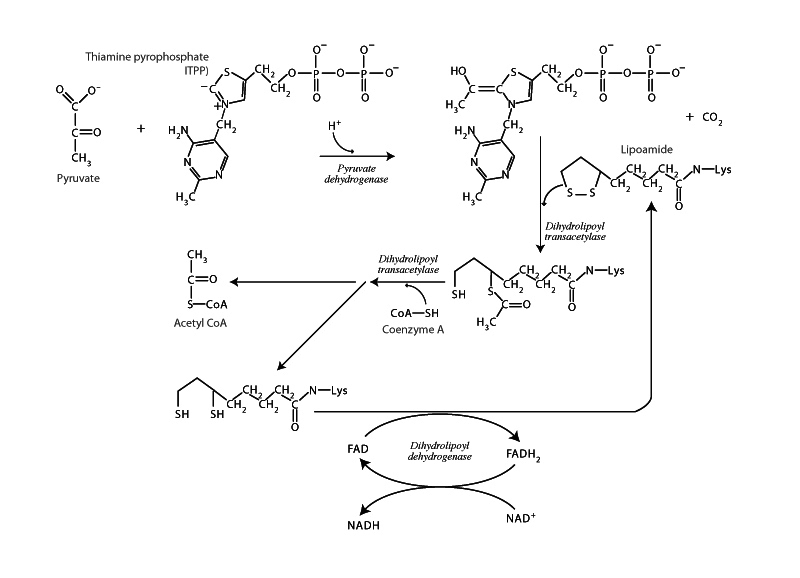
Although glycolysis takes place in the cytoplasm of eukaryotic cells, the oxidative decarboxylization of pyruvate to form acetyl CoA and, later, the citric acid cycle take place within the mitochondrion. The oxidative decarboxylization of pyruvate is catalyzed by the pyruvate dehydrogenase complex and carries out the net conversion of pyruvate, CoA, and NAD+ into acetyl CoA, CO2, and NADH.
Careful attention the pyruvate dehydrogenase complex mechanism yields a great deal of insight into recurrent themes in biochemistry and fundamental ideas about energy metabolism. Keep in mind the overall plot of the story. Electrons are being extracted from the nutrient molecule glucose ultimately to be passed to the acceptor, which is oxygen.
This reaction could take place all at once. We could combust glucose on the bench-top, and a great deal of heat would flow into the surroundings. Oxygen pulls the electrons that used to be in covalent bonds between carbon and carbon and carbon and hydrogen in towards itself in CO2 and H2O bonds; the internal energy of the system decreases along lines of electrostatic potential energy; and heat flows into the surroundings. The heat flowing into the surroundings is increasing the entropy of the surroundings. The surroundings are part of the universe. The heat flow is increasing the entropy of the universe. The heat flow is not going to turn around in reverse and put the glucose back together. Combustion is spontaneous and irreversible. There is also an increase in the entropy of the system too - it should be mentioned - because there are more moles of gas, so everything, system and surroundings, is pointing towards combustion of glucose being spontaneous. We conveniently encapsulate the combined effect on system and surroundings when we say the free energy of the system is decreasing.
In metabolism, the process is not happening all at once, though, but in small enzymatic stages through the steps of glycolysis, pyruvate dehydrogenase complex, citric acid cycle, and electron transport. Just like the working body of an engine couples spontaneous heat flow to productive work, the enzymatic pathways of energy metabolism couple the spontaneous oxidation of glucose with the nonspontaneous formation of ATP. The catabolism of glucose occurs through step-wise processes. Just like the Carnot cycle, the stages are often as near to equilibrium as possible, but metabolism must be overall spontaneous to keep it moving forward. ΔG for combustion of glucose is about -3000 kJ. Forming 36ATP is about +1100kJ.
Through pyruvate dehydrogenase and the citric acid cycle in the mitochondrion, electrons, which had been the property in redox terms of carbon in glucose, are being passed to the electron carriers NAD+ and FAD. Those will still be holding them up at a high energy. They aren't falling down into a well like they would with oxygen. You can see almost a kind of care taken in the steps of the pyruvate dehydrogenase complex to move the electrons onwards without losing heat flow to the surroundings.
The coenzyme thioamine pyrophosphate (TPP) doesn't actually oxidize the glucose when it cleaves it, liberating CO2. Although the carbon in CO2 now has a +4 oxidation state, which in pyruvate had been +3, control of the electron it lost actually passed to the carbon now attached to TPP. It's oxidation number changed from +2 to +1. The coenzyme that actually removes the electrons from the nutrient carbon will be lipoamide in the next step. When TPP passes the two carbon fragment to lipoamide, the oxidation number of that same carbon changes from +1 to +3, and each of the two sulfurs of lipoamide change in oxidation state from -1 to -2. Two electrons have been transferred from the nutrient carbon, one to each of the sulfur atoms. Carbon has electronegativity of 2.5. Sulfur's is 2.6. This is a nearly level shift in terms of standard reduction potential. The electrons haven't fallen down into a well. They're being held by sulfur at nearly the same energy as in the nutrient.
Take a moment to appreciate that the oxidized form of lipoamide is the disulfide form; the reduced form is the sulfhydral form. From lipoamide to disulfide bridges in proteins to glutathione, the major redox buffer in the blood, the redox cycling of sulfur between these two forms, disulfide and sulfhydral, is a crucial theme that will keep getting more and more important the more you learn about biochemistry. Understanding this is a big figure of merit for the MCAT.
So sulfur now has the electrons, which it will then pass to FAD, and then FADH2 passes them to NAD+. When both of those substances receive electrons, they lose aromaticity, the isoalloxazine ring system in FAD and the nicotinamide ring in NAD+. This is why the electrons aren't falling down into a well here either. NAD+ and FAD both have negative standard reduction potential - meaning that electrons would have an uphill climb to get onto those from a standard hydrogen electrode. FADH2 and NADH both want to give the electrons away so that they can be aromatic again. NADH, of course, will be able to do that at complex I (NADH ubiquinone oxidoreductase) of the electron transport chain. It's through the electron transport chain that the electrons will fall in energy as they make their way towards the positive reduction potential of oxygen, their fall coupled with pumping protons into the outer compartment of the mitochondrion for the subsequent powering of ATP synthase.