Interdisciplinary Note (4 of 9)
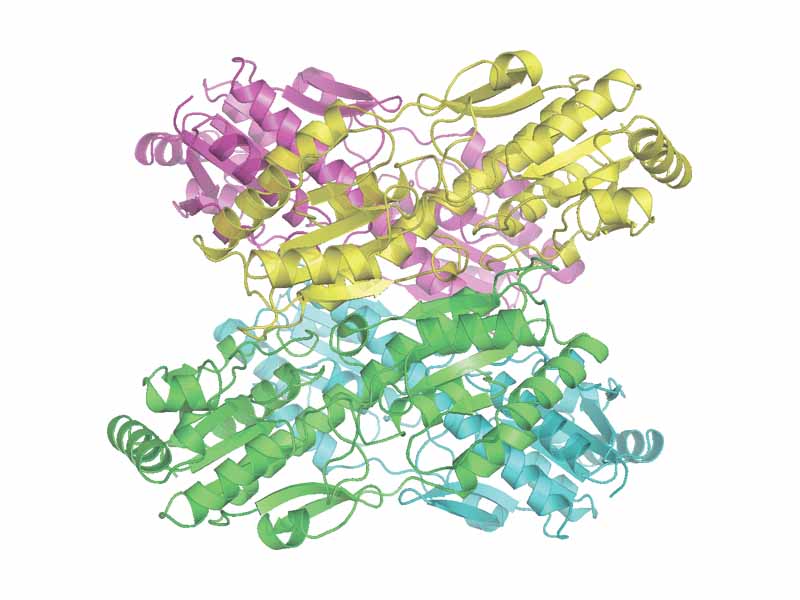
Phosphofructokinase I is a multi-subunit, allosteric enzyme. PFK I is a tetramer, ike hemoglobin, and like hemoglobin, PFK I is a dimer of a dimer. Each dimer of PFK I contains a substrate binding site as well as a separate sites for binding of allosteric inhibitors and promoters. PFK I is the committed, rate-determining step for glycolysis. It is a dogma of biochemistry that the committed step in a biochemical pathway will be the primary control point for regulation, and regulation converges on this enzyme from many directions.
Firstly, eEnergy charge is a primary regulatory factor. ATP inhibits PFK I. In other words, when energy charge in the cell is high, glycolysis will be slowed down, but when energy charge is low, glycolysis is turned up. AMP is a strong promoter of PFK I. In fact, AMP is such a strong promoter that the enzyme loses cooperativity because both substrate binding sites will already be in a completely open configuration. The reaction velocity vs. substrate concentration curve will no longer be sigmoidal but takes on the hyperbolic shape of Michaelis-Menten kinetics.
PFK I is also promoted by the substance fructose-2,6-bisphosphate. What does fructose-2,6-bisphosphate have to do with anything? This is always confusing at first. Fructose-1,6-bisphosphate, not fructose-2,6-bisphosphate, is the product of the PFK I reaction. Fructose-2,6-bisphosphate is produced by a different enzyme, PFK II. PFK II produces fructose-2,6-bisphosphate as a kind of "on switch" for PFK I. This is a mechanism for endocrine control to reach in and affect the metabolic profile of the cell. It can allow a high energy charge, high ATP, in other words, to be overridden and glycolysis to occur anyway. For example, under insulin, gluconeogenesis in a liver cell will be off, of course, and glycolysis will be turned on, but the reason to run glycolysis won't be to make ATP, so much, but to feed glucose through glycolysis and pyruvate dehydrogenase to make acetyl CoA for fatty acid synthesis. Insulin activates a protein phosphatase which dephosphorylates the PFK-2 complex and causes PFK-2 activity. PFK-2 then increases production of fructose-2,6-bisphosphate to turn on PFK-1. Voila!