Interdisciplinary Note (6 of 9)
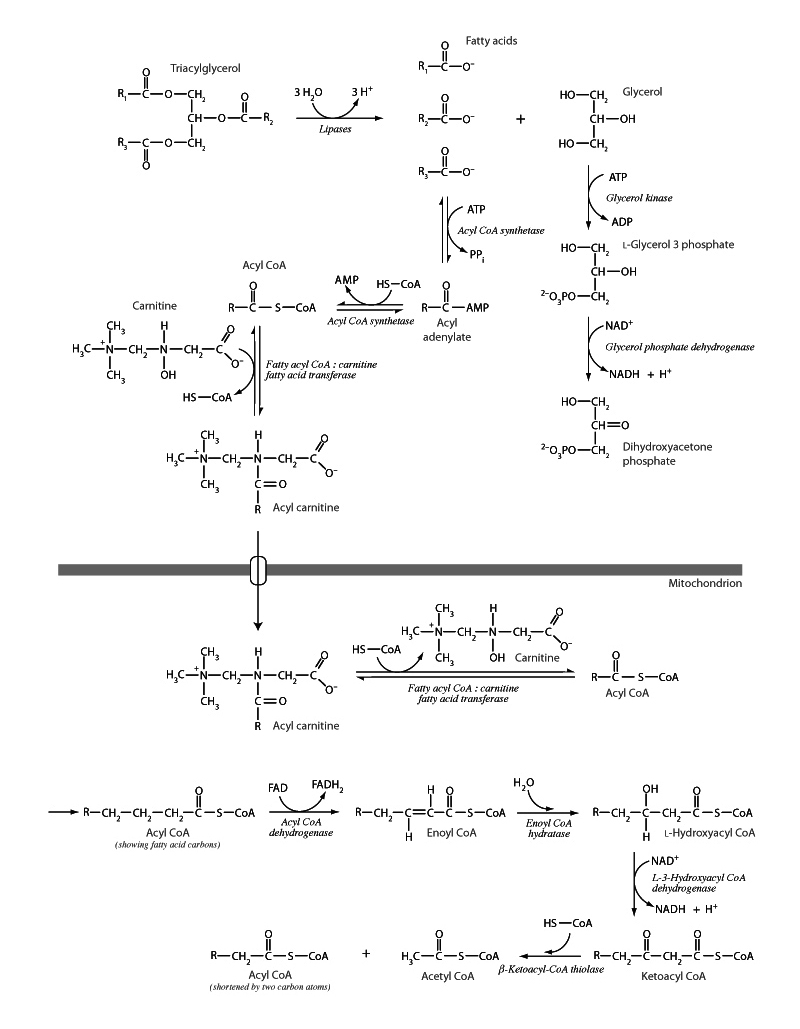
A few key insights make the pathway in β-oxidation easy to understand and remember. Firstly, there is the organic chemistry rationale of the pathway. Highly reduced carbon has been stored in triglyceride. After lipolysis, activation of the fatty acid as fatty acyl CoA, and shuttling to the mitochondrion, the fatty acid needs to be broken down two carbon units at a time to liberate acetyl CoA for the citric acid cycle - or, alternatively, ketone body synthesis. In order to carry this out, some work needs to done on the β-carbon of the fatty acyl CoA. It needs to be turned into a carbonyl group. It needs to be oxidized, in other words. A carbonyl at the β position will make possible a retro-Claisin mechanism - Claisin condensation backwards. This is how β-ketothiolase will break off and liberate acetyl CoA. Forward Claisin condensation yields a β-keto ester. That's what ketoacyl CoA is, though technically a β-keto thioester. Ketoacyl CoA can do Claisin condensation backwards. In summary, remember the point of the pathway, we need to put a carbonyl at the β position to set up a retro-Claisin cleavage to liberate acetyl CoA.
The second insight that makes β-oxidation easy to understand and remember is that the three steps which convert the fatty acyl CoA into ketoacyl CoA are directly analogous to three steps in the citric acid cycle. Acyl CoA dehydrogenase uses FAD to oxidize the fatty acid, forming a double bond between the α and β carbons in enoyl CoA. This is analogous to succinate dehydrogenase in the TCA using FAD to oxidize succinate to form the double bond in fumarate. Next, the double bond is hydrated in β-oxidation by enoyl CoA hydratase to form hydroxyacyl CoA. In the TCA, fumarate is hydrated by fumarase to form malate. In the third step in β-oxidation, hydroxyacyl CoA dehydrogenase, oxidation by NAD+ converts the hydroxyl group into the carbonyl group to form the ketoacyl CoA discussed above. This step is analogous to malate dehydrogenase oxidizing malate to form NADH and oxaloacetate in the citric acid cycle. In other words, if you can remember one, you can remember the other. Oxidation with FAD to form a double bond. Hydration of the double bond to form an alcohol. Oxidation of the alcohol to by NAD+ to form a carbonyl.