Interdisciplinary Note (3 of 9)
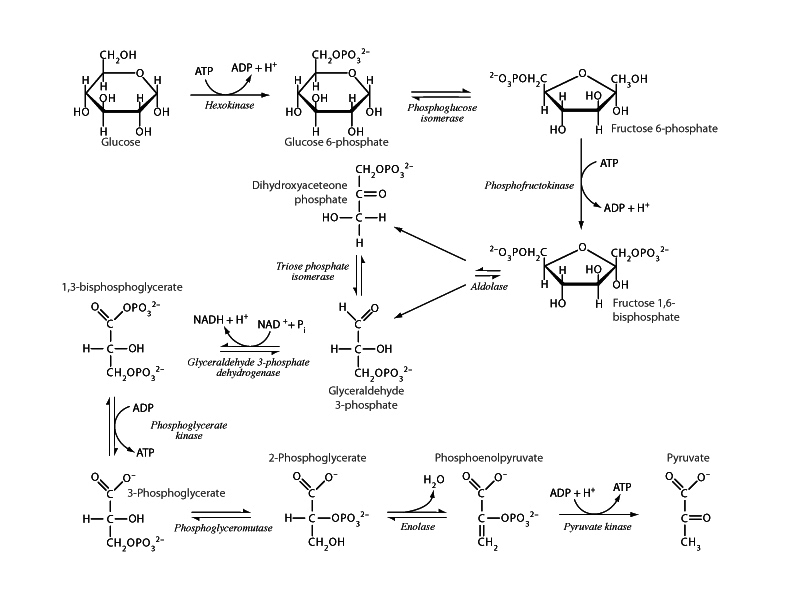
Only three steps in glycolysis have a large negative free energy change under physiological conditions. These steps are hexokinase, phosphofructokinase I, and pyruvate kinase. Remember these, because those reactions will be different in gluconeogesis. The steps in a pathway with large negative free energy are where regulation converges, especially the committed step. PFK I is the committed step in glycolysis. When regulation turns these enzymes on, it's like a firehose that pushes all the other steps forward.
Another reaction in glycolysis, phosphoglycerate kinase, actually does have a large negative standard free energy change. However, in the cytosol the concentrations of 1,3-bisphosphoglycerate and 3-phosphoglycerate are near the equilibrium point (ΔG0 = -18.8 kJ/mol, ΔGphys = 0 to 2 kJ/mol).
It is so important for this exam that you remember that the standard free energy change predicts the direction of a reaction for a state in which the concentration of product and reagent are both equal to 1M. The actual free energy change may be different than that. The actual free energy change depends on the actual concentrations as reflected in the reaction quotient, how much product and reagent are actually there. If there is a high concentration of product and very little reagent, a reaction with a high negative standard free energy change could even go backwards.
In fact, when flux is in the direction of gluconeogenesis, phosphoglycerate kinase gets pushed backwards under Le Chatelier&##9;s principle by the constant appearance of 3-phosphoglycerate through phosphoglucomutase which itself is being pushed backwards. Phosphoglycerate kinase moves forward by mass action under glycolysis and backwards by mass action under gluconeogenesis.