Interdisciplinary Note (2 of 9)
Many of the steps of the biochemical pathways that are required knowledge for the new MCAT involve organic reaction mechanisms for which you are also responsible. In fact, it's almost certainly true that the primary rationale for an organic reaction's selection for inclusion in the MCAT outline is its importance in biochemistry. There are only a few possible exceptions, such as organosilane protecting group, likely included because of importance in pharmacological organic synthesis and the molecular biology benchtop. On the exam, you are much more likely to see an organic chemistry reaction embedded within the complexities of the biochemical context than as an organic chemistry benchtop reaction.
Just moving through the first few reactions of glycolysis, we can see how this works. Beginning with the hexokinase step, we have phosphorylation of glucose by ATP. Phosphoryl transfer mechanism is, unfortunately, under-emphasized in traditional organic lecture class. The basic phosphoryl transfer mechanism is just like SN2 substitution, but across a phosphorus, not carbon, with backside attack on one side and the leaving group departing from the other. Picture the C6 hydroxyl group of glucose as a nucleophile attacking the γ-phosphate with ADP leaving from the other side.
The next step in glycolysis is phosphoglucose isomerase (phosphohexose isomerase), which interconverts glucose-6-phosphate and fructose-6-phosphate. This mechanism involves a crucial organic concept for the exam, keto-enol tautomerism. The intermediate form in the mechanism is an enol. It's actually an enol tautomer of both glucose-6-phosphate and fructose-6-phosphate. It tautomerizes from glucose into the enol and from the enol into fructose, and vice-versa.
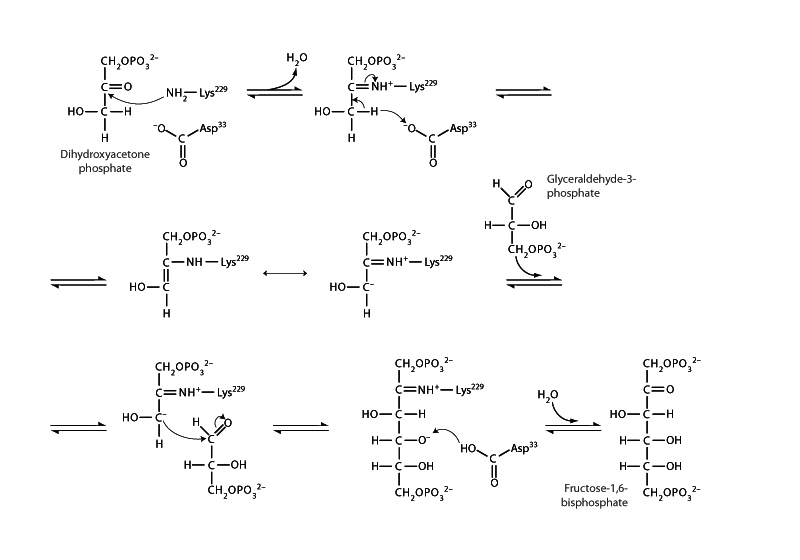
In the next step we have a 2nd phosphorylation by ATP in phosphofructokinse I (PFK I) resulting in fructose-2,6-bisphosphate, and then this is followed by the aldolase step to form glyceraldehyde-3-phosphate and dihydroxyacetone phosphate. Textbooks are fond of saying that the reaction is a retro-aldol addition (aldol addition in reverse). This is in the glycolysis direction. In gluconeogenesis, the reaction happens in the aldol addition direction. However, the aldolase mechanism is not exactly an aldol mechanism, but more aldol-like. In the gluconeogenesis direction, the mechanism begins with imine formation between enzyme lysine and dihydroxyacetone phosphate. Imine formation is another MCAT reaction! Just like a ketone can interconvert with its enol tautomer, an imine can interconvert with its enamine tautomer, so it's not an enolate anion carrying out aldol addition, but the analogous imine-enamine form, called an iminium anion. In glycolysis this is happening backwards. Retroaldol cleavage to liberate glyceraldehyde-3-phosphate from the DAP-lysine Schiff base. (Schiff base is what a biochemist calls an imine.) After imine hydrolysis (the reverse of imine formation) the dihydroxyacetone phosphate is also liberated.
The next step, triose phosphate isomerase interconverts G3P and DAP. In the glycolysis direction, product removal drives the reaction forward to G3P. Triose phosphate isomerase mechanism is identical in nature to phosphoglucose isomerase. DAP tautomerizes to an enol and then tautomerizes out of the enol to G3P.
The very next step, glyceraldehyde-3-phosphate dehydrogenase is yet another example of an MCAT reaction, oxidation of an aldehyde. You get the picture.
Well, this was a difficult interdisciplinary note, but it needs to be intelligible. It's a good sign if you were able to read it comfortably, both for the biochemistry and organic chemistry.