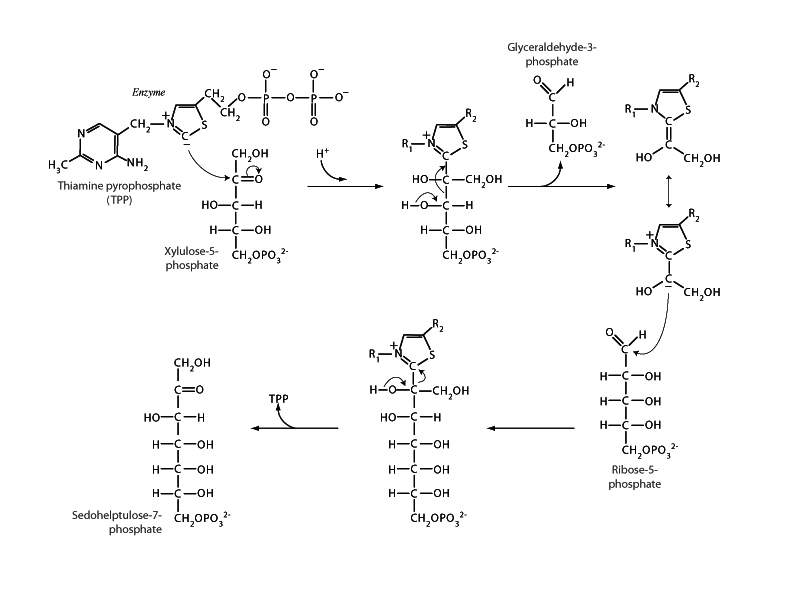
The overall process of the non-oxidative phase of the pentose phosphate pathway is to convert three C5 sugars into two C6 sugars and one C3 sugar. Transketolase carries out the first of these reactions with transaldolase carrying out the second reaction and transketolase appearing again to carry out the third. In the first transketolase reaction, shown above, the cofactor thiamine diphosphate accepts a 2-carbon fragment from the 5-carbon ketose, xylose-5-phosphate, leaving the 3-carbon glyceraldehyde-3-phosphate. Transketolase then transfers this fragment to the 5-carbon aldose, ribose-5-phosphate, to form a 7-carbon ketose, sedoheptulose-7-phosphate.
A recurrent motif in biochemistry is that enzymatic reactions in which carbon-carbon bonds are broken or formed will often involve a stabilized carbanion intermediate. Such a carbanion can serve as a nucleophile to add to the electropositive carbonyl carbon of a substrate. In the transketolase mechanism, the ylide* form of the cofactor thiamine pyrophosphate is used to form such an intermediate in joining with the two-carbon fragment removed from xylulose-5-phosphate, a resonance stabilized imine-enamine carbanion intermediate, which can next perform nucleophilic addition upon the aldehyde group of ribose-5-phosphate forming sedoheptulose-7-phosphate.
* An ylide is a compound with positive and negative charges on adjacent atoms.