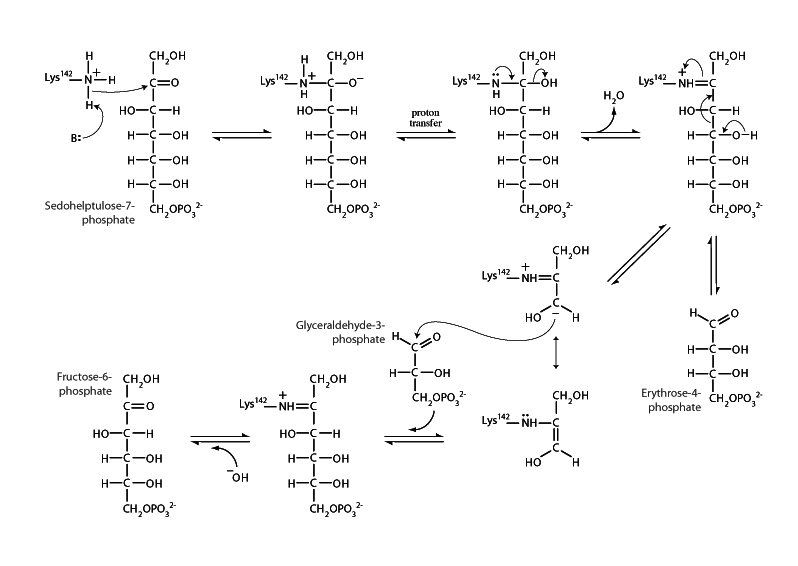
Transaldolase is an enzyme of the non-oxidative phase of the pentose phosphate pathway. Transaldolase catalyzes the transfer of a 3-carbon unit from sedoheptulose-7-phosphate to glyceraldehyde-3-phosphate. This yields erythrose-4-phosphate and fructose-6-phos- phate. The transaldolase mechanism begins with a lysine residue forming a Schiff base with sedoheptulose-7-phosphate. Formation of this Schiff base creates the conditions for a variation of retro-aldol cleavage to occur in which a 3-carbon portion is broken from sedoheptulose-7-phosphate as an enzyme bound resonance stabilized imine-enamine intermediate with the remainder released as erythrose-4-phosphate.
Formation of this Schiff-base stabilized intermediate is the vehicle for the enzyme to move the 3-carbon portion from sedoheptulose-7-phosphate to glyceraldehyde-3-phosphate. The intermediate is resonance stabilized and in possession of a nucleophilic carbon that can undergo addition to the glyceraldehyde-3-phosphate carbonyl group. Nucleophilic addition to glyceraldehyde-3-phosphate forms enzyme bound fructose-6-phosphate. The Schiff base link between the enzyme and fructose-6-phosphate is next broken by hydrolysis, regenerating transaldolase and releasing fructose-6-phosphate.