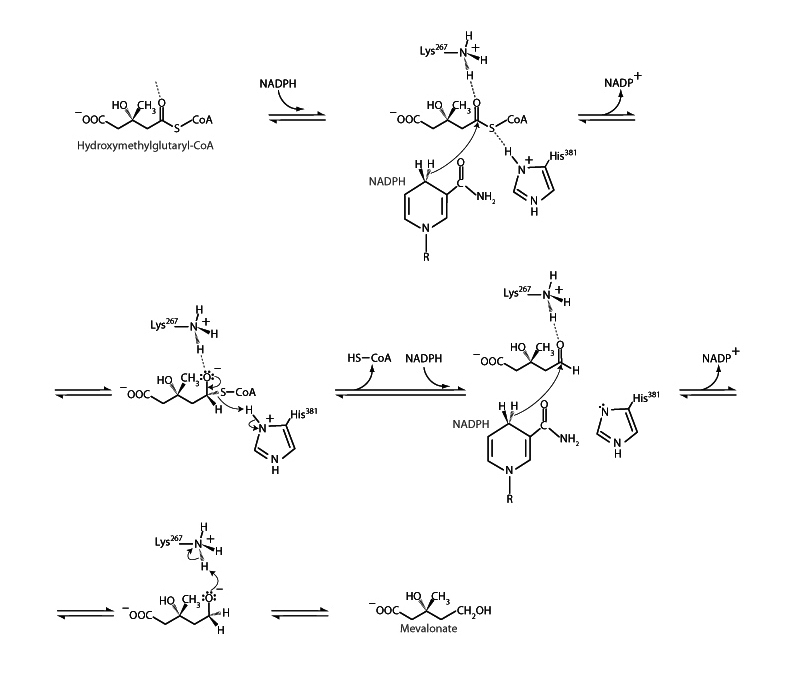
Hydroxymethylglutaryl-CoA (HMG-CoA) is the precursor of isopentenyl pyrophosphate, which is the build- ing block of the isoprenoid lipids as well as steroids.* HMG-CoA is reduced to mevalonate to start the pathway to isopentenyl pyrophosphate. The HMG-CoA reductase mechanism is similar to the organic benchtop reduction of a carboxylic acid to an alcohol with LiAlH4. Reduction occurs in two stages, first forming an aldehyde then forming the primary alcohol. The HMG-CoA reductase reaction begins with a hydride (H-) from NADPH attacking the carbonyl of the HMG-CoA thioester to form a tetrahedral intermediate. The tetrahedral intermediate collapses with departure of the thiol group, forming an aldehyde. Hydride from another NADPH adds to the carbonyl group of the aldehyde. Electrons from the carbonyl group move up onto the carbonyl oxygen to form an alkoxide. Protonation of the alkoxide creates the primary alcohol of mevalonate.
* Steroid synthesis is just past the borderline of required knowledge for the MCAT. It's helpful to have a good general sense about this material, but you don't need to anticipate questions on specifics. Here, the focus is on the behavior of NADPH which IS required.