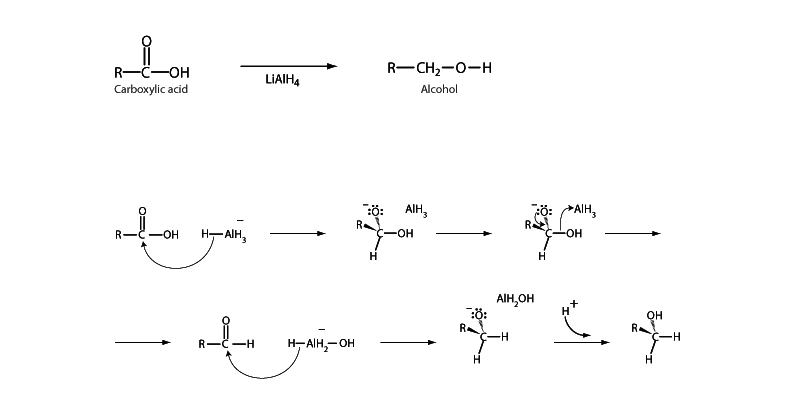
Carbohydrates can be reduced to primary alcohols by lithium aluminum hydride (LiAlH4). The reaction happens in two stages, first forming an aldehyde then the primary alcohol. The reaction begins with a hydride (H-) from LiAlH4 acting like an acyl substitution nucleophile, attacking the carbonyl of the carboxylic acid to form a tetrahedral intermediate. The tetrahedral intermediate collapses with departure of the hydroxyl group. An aldehyde is formed. However, the aldehyde does not persist because aldehydes themselves may be reduced. Another hydride anion adds to the carbonyl group of the aldehyde with electrons from the carbonyl group moving up onto the carbonyl oxygen to form an alkoxide. Protonation of the alkoxide creates the primary alcohol.