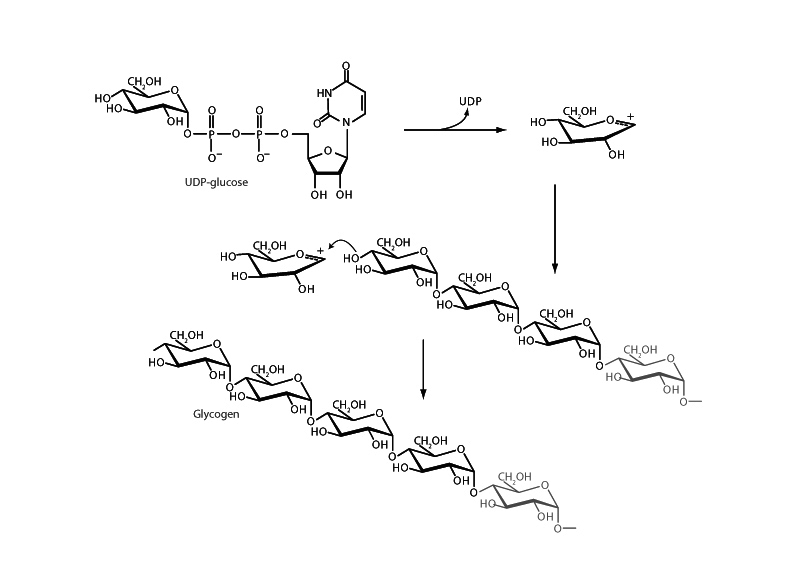
The formation of the glycosidic linkage between two carbohydrate moeities is an instance of the second part of acetal formation in biochemistry in which an alcohol adds to a hemiacetal. With reference to acetal formation, the second sugar's anomeric carbon begins the process at the hemiacetal stage. The cyclic form of a sugar is a hemiacetal. In glycosidic bond formation, the hydroxyl group of one sugar carries out nucleophilic addition upon the hemiacetal anomeric carbon of another. Before this carbon can receive the nucleophile, this anomeric carbon must be transformed into an oxonium cation through departure of its hydroxyl group. However, hydroxide is a poor leaving group. In benchtop acetal formation, oxonium cation formation occurs through acid catalyzed dehydration. In the biochemical context, however, such significantly acidic conditions are not feasible, so instead, the hydroxyl group must first be transformed into a good leaving group.
Glycogen synthesis includes such a strategy. In this pathway, the UDP moeity is positioned by UDP-glu- cose pyrophosphorylase, extending from the phosphate of glucose-1-phosphate, to form an excellent leaving group at the C-1 position. Activating glucose in this way enables oxonium cation formation through UDP departure in glycogen synthesis. The substrate is then prepared for nucleophilic attack and the formation of the glycosidic linkage between the anomeric carbon and the non-reducing end of the growing glycogen chain.