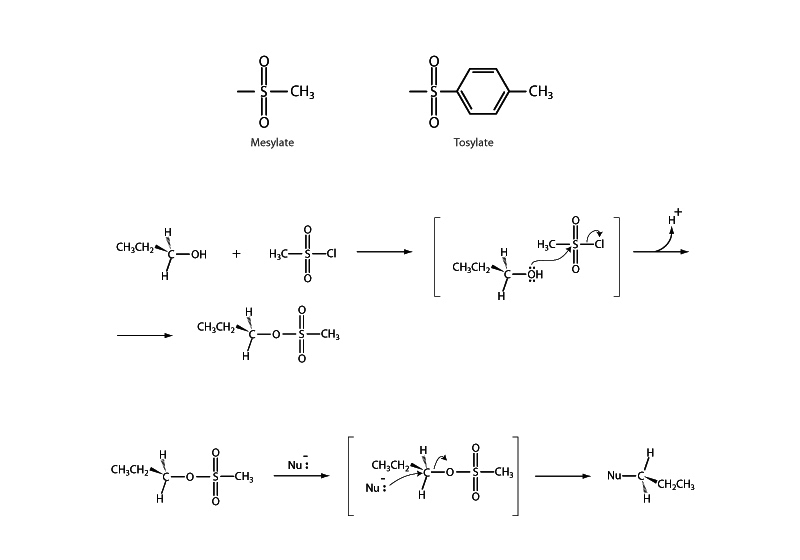
What makes a good leaving group? When a leaving group departs from a substrate in a substitution reaction, it takes an electron pair with it. How stable will the leaving group be after it departs? Can the negative charge spread out or does it remain compressed within a small region? This type of discussion of internal energy is similar to the one underlying the determination of the position of equilibrium for an acid-base couple. Will the conjugate base be very stable? If so, the acid will be strong. In fact, as a general rule of thumb the best leaving groups are also very weak bases. Strong bases are poor leaving groups. Weak bases are substances that can manage as negative charges in solution without taking on high energy.
This presents a problem for substitution reactions involving alcohols. Because hydroxide is a strong base it makes a poor leaving group. This problem can be circumvented with acid catalysis, but when that isn't practical, another solution to the problem is by turning the alcohol into a sulfonic ester. A commonly employed method being either to form an organic mesylate or an organic tosylate by treatment of the alcohol with either methanesulfonyl chloride or para-toluene sulfonyl chloride. Mesylate and tosylate groups are excellent leaving groups in nucleophilic substitution reactions because the negative charge on the leaving group is stabilized by resonance.