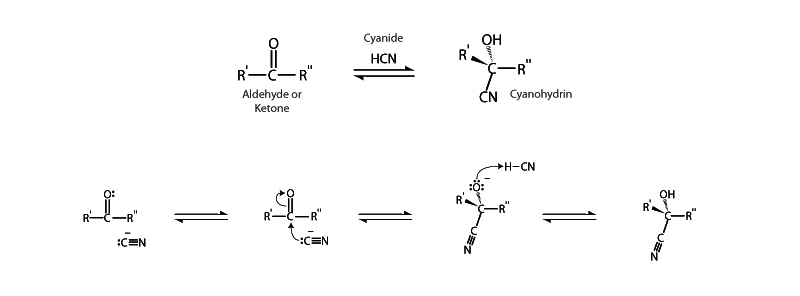
The cyanohydrin functional group possesses both a hydroxyl and a cyano group bonded to the same carbon atom. Cyanohydrins are produced through the reversible nucleophilic addition of cyanide anion (CN–) to an aldehyde or ketone. The nucleophilic cyanide anion attacks the carbonyl carbon on the ketone to form the stable, tetrahedral cyanohydrin.
*A cyanide nucleophile is similarly employed in the Strecker amino acid synthesis (pg 117), but to attack an imine.