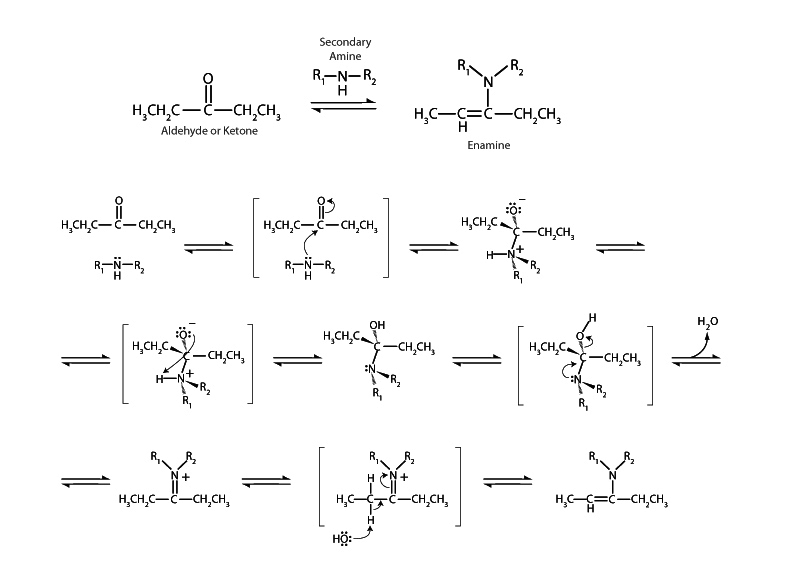
Secondary amines react with aldehydes and ketones to form enamines. In the case of a primary amine nucleophile an imine is formed instead (pg 6). In imine formation an original hydrogen remains from the primary amine on the iminium cation after addition and hydrolysis, and this nitrogen-hydrogen bond can be broken heterolytically to satisfy the iminium cation's electron deficiency. With enamine formation, however, where the nucleophile is a secondary amine not a primary amine, there is no such nitrogen-hydrogen bond at the iminium cation stage. The iminium cation intermediate must look elsewhere for an electron source to satisfy its thermodynamic drive to reduce internal energy.
The carbon-hydrogen bond at a position alpha to carbon-nitrogen double bond is an acceptable electron source. After that bond is broken, electron density can flow by conjugation to satisfy the iminium cation. This occurs by formation of a carbon-carbon double bond interior to the alpha carbon which causes the carbon-nitrogen double bond on the other side to become a single bond, releasing an electron pair to become the sole property of nitrogen. Between carbon and nitrogen there is now a single bond and the nitrogen atom is neutral. These electron movements are very similar to the electron movements that occur in keto-enol tautomerism (pg 23). It can be productive to think of enamines as the nitrogen-carbon cousins of enols. (See imine-enamine tautomerism, pg 31.)