Interdisciplinary Note (3 of 11)
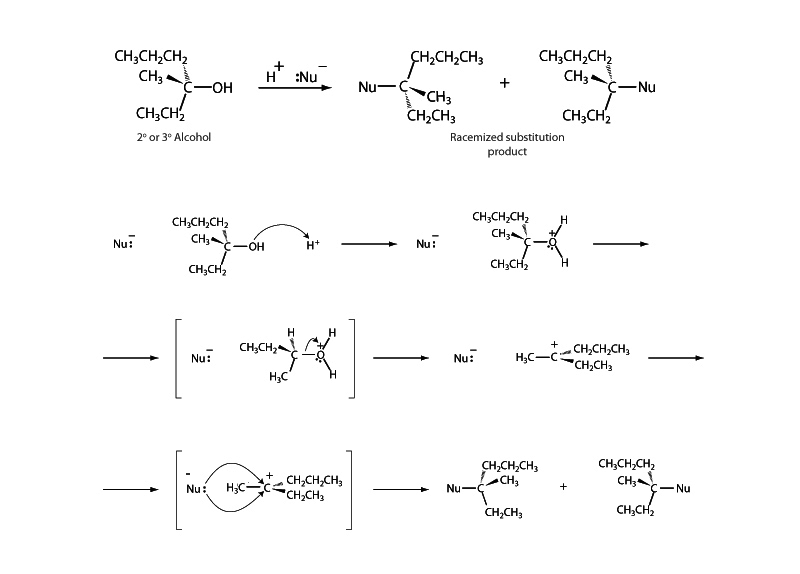
It might be a bit confusing to hear about an SN1 substitution involving an alcohol in which the rate is described as depending upon both the concentration of the alcohol substrate and the concentration of hydrogen ion H+. How could a first order reaction depend on the concentration of two things?
The issue is that H+ is not a reagent in the reaction. It is a catalyst. Acidic conditions facilitate substitution reactions involving alcohols because the hydroxyl group is such a poor leaving group. Protonation enables the group to leave as water.
Does [H+] appear in the rate expression? Not directly but indirectly. A catalyst lowers activation energy. While [H+] does not appear in the rate expression, the hydrogen ions lower the value of the rate constant. You can see how this works in the Arrhenius equation, k = Ae-Ea/RT