Interdisciplinary Note (2 of 11)
The reaction order is the relationship between the concentrations of species and the rate of a reaction. The order of a reaction cannot be predicted from the overall stoichiometric equation. You would either need to know the path the reaction follows, its mechanism, or you need to gather data from experiment to determine the reaction order. The rate expression for the reaction will agree with the stoichiometric equation only if all species are present in stoichiometric ratio at the rate determining step of the reaction mechanism.
The reaction mechanism determines the rate expression, not the overall stoichiometry. If a reagent is not present at the rate determining step, its concentration will not appear in the rate expression. Don't start putting stoichiometric coefficients into a rate expression out of habit gained from reaction quotients and equilibrium constants. This is the oldest multiple choice trick in the MCAT book. Don't fall for it! To determine the rate expression, you need to know the mechanism or have experimental data.
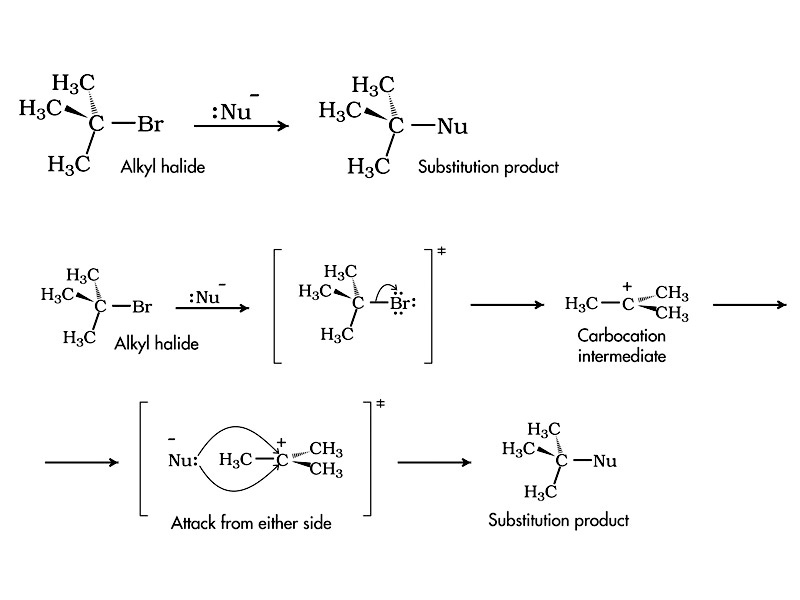
Substitution and elimination reactions are the quintessential example from organic chemistry of this pitfall. The stoichiometry of SN1 versus SN2 substitution looks the same, but the former is 1st order and the latter is 2nd order. If the rate of the reaction varies with the concentrations of both the substrate and the nucleophile, the mechanism must be bimolecular SN2. However, if only changing the concentration of substrate changes reaction rate, and the rate is indifferent to nucleophile concentration, the mechanism must be unimolecular SN1. The mechanisms are different. In SN2 substitution, both the substrate and nucleophile are present in the rate determining step, but with SN1 substitution, the rate determining step is carbocation formation. Because only the substrate is present in that step, only the substrate appears in the rate expression.