Interdisciplinary Note (10 of 29)
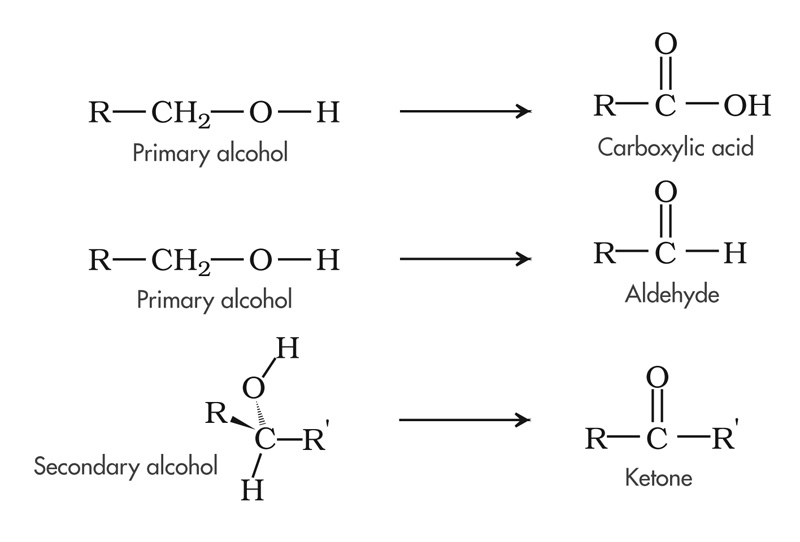
Carbon is in a progressively more oxidized state from alkanes to alcohol to aldehyde/ketone to carboxylic acid to carbon dioxide. Progressing up the scale involves a continuous transfer of 'electron control' from carbon to oxygen. Because oxygen (3.5 on the Pauling scale) is more electronegative than carbon (2.5), in terms of oxidation-reduction, electrons shared between the two atoms within covalent bonds are said to belong to oxygen. At the aliphatic end of the scale, carbon is bonded to other carbons or hydrogen. At the oxidized end, carbon is bound to oxygen. A carbon in a primary alcohol is in a relatively reduced state, with an oxidation number of -1. In the primary alcohol, the carbon has gained two electrons from bonding with two hydrogens and it has lost one electron in bonding to oxygen. This carbon would have an oxidation state of +1 upon oxidation to an aldehyde and +3 upon further oxidation to carboxylic acid.