Interdisciplinary Note (5 of 11)
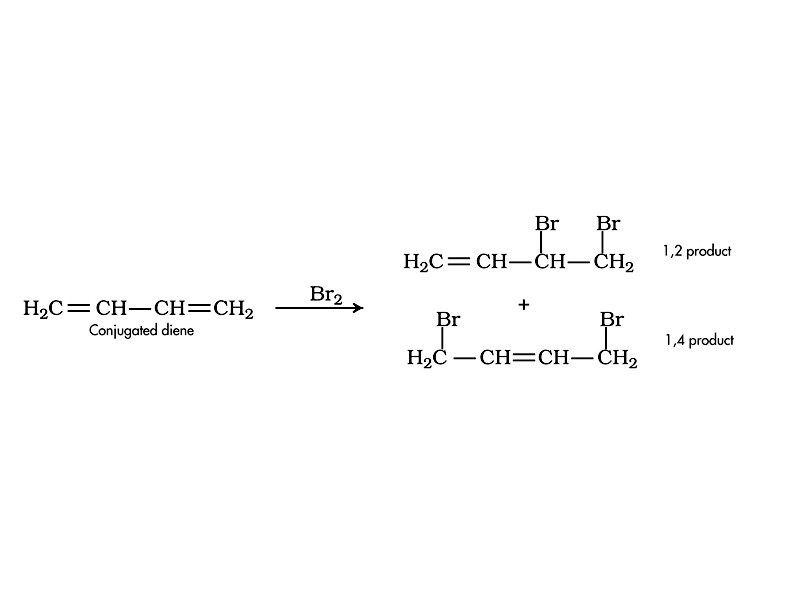
Addition of halogen to a conjugated dienes is the classic example involving the interplay of kinetics and thermodynamics in determining the nature and extent of reaction yield. When Br2 is reacted with a conjugated diene at low temperatures, direct addition (1,2 addition) is favored. However, at room temperature and above, conjugate addition (1,4 addition) is favored. This occurs because the 1,2 product is kinetically favored but the 1,4 product is thermodynamically favored.
Why is one pathway kinetically favored and the other thermodynamically favored? The activation energy is greater for the conjugate addition pathway (1,4 addition), so it occurs more slowly than 1,2 addition. However, the product of 1,4 addition contains an internal double bond, leading to lower free energy than the 1,2 product. Therefore, 1,4 addition is thermodynamically favored.
When the addition is carried out under conditions such that the products may equilibrate (higher temperature), the product composition no longer reflects the relative rates of formation but, instead, thermodynamic stability, so the 1,4 product, having lower free energy, is the major product. At low temperature, on the contrary, only a vanishingly small fraction of the reagent has sufficient energy to overcome the activation energy barrier to form the 1,4 product, so the 1,2 product is the major product. The 1,4 product is the thermodynamic product, while the 1,2 product is the kinetic product.
Electrophilic addition to conjugated diene is the classic example from organic chemistry lecture of the concept of thermodynamic versus kinetic control. Another good example involves an aldol addition reaction where the α-carbons on either side of the carbonyl group differ in their degree of substitution. Recall that aldol addition involves an enolate anion intermediate, formed by departure of an α-hydrogen. Because there is an α-hydrogen on either side of the carbonyl, the reaction has a choice as to which proton will depart in enolate formation. Recall that one of the resonance forms of an enolate possesses a double bond between the original carbonyl carbon and the α-carbon. Because greater substitution thermodynamically stabilizes a double bond, the free energy of the resonance form with the more highly substituted α-carbon will be lower. In such reactions, the aldol addition product formed through formation of the more highly substituted choice of enolate will be the thermodynamic product. The other will be the kinetic product.
Kinetic versus thermodynamic control is an important concept in organic chemistry class, but why does the MCAT care about these ideas? At first glance, kinetic versus thermodynamic control seems like an issue for benchtop chemistry, not biochemistry. The prevailing ethos governing the organic chemistry focus on the MCAT is to emphasize the organic chemistry most important to biochemistry and molecular biology. However, it would be hard to find a set of concepts more centrally important in living systems than kinetic versus thermodynamic control.
Metastability is a stable state of a dynamical system other than the system's state of least energy. A ball resting in a hollow on a slope is a simple example of metastability. If the ball is only slightly pushed, it will settle back into its hollow, but a stronger push may start the ball rolling down the slope. A misfolded protein is like the ball resting in the hollow on the slope. A misfolded protein is stuck in a kinetic trap. In kinetic versus thermodynamic control, the kinetic product is a metastable state of the system.
While the native state of a protein represents a free energy minimum, many of the 'proper' forms of biological molecules represent metastable states. A foremost example is ATP, which is kinetically stable but not thermodynamically stable. The cell itself is metastable. The kinetically controlled processes of self-assembly and self-replication in cellular structure operate under non-equilibrium conditions. A living cell is a dynamic, metastable system working far-from-equilibrium, determined by kinetic over thermodynamic control.