Interdisciplinary Note (9 of 12)
Why doesn't chemistry refer to heat flow simply as 'heat flow'? Why 'enthalpy change'? Heat is not an inherent property or condition of a system, so it is not proper to refer to 'heat energy'. Heat refers to a transfer of energy. However, by specifying constant pressure, we can conceptualize a new state function for the system, the enthalpy, H, where H = U + PV. Enthalpy is a state function whose change equals the heat flow (as long as pressure is constant). When you say enthalpy change, you generally do mean heat flow, but you are saying something else besides. You are expressing a change in a state function. Enthalpy change is path independent.
To say that enthalpy change equals heat flow is simply the 1st law of thermodynamics, i.e. the heat flow results from the change in internal energy and work. What you are referring to when you refer to enthalpy in itself is a property of the system that includes the internal energy plus the work involved to push back the environment to create the space the system occupies. Enthalpy can be thought of as the 'thermal potential' of the system.
Enthalpy is a pretty good stand-in for internal energy in chemical discussion. It differs from internal energy in that when it changes, heat flows by the same amount, but the enthalpy change equals internal energy change if the volume is constant, which, much more often than not, applies in biochemistry.
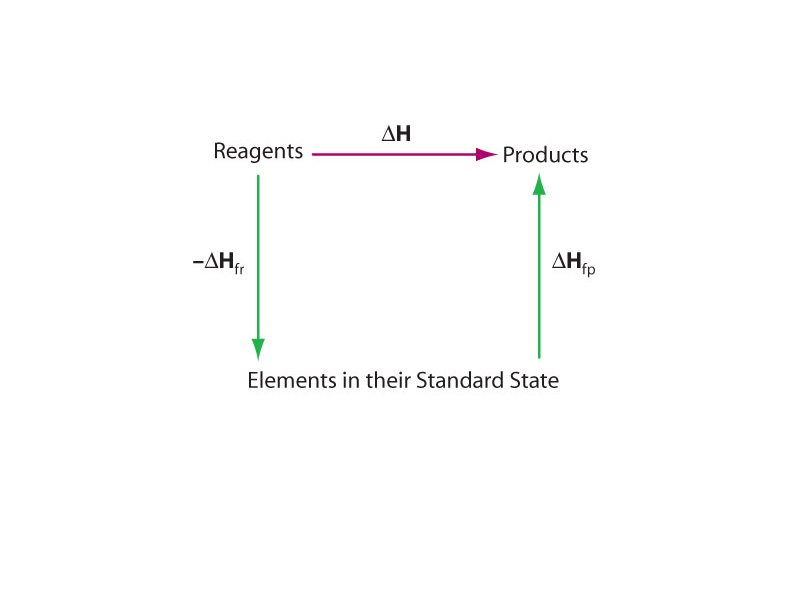
The big advantage that comes from describing heat flow in terms of enthalpy change in chemistry is that, because enthalpy is a state function, the difference in enthalpy doesn't depend on the path between two states of the system. In other words, describing heat flows in terms of enthalpy change allows us to use Hess' Law of Heat Summation.
Chemical thermodynamics is an edifice built on state functions. Internal energy, enthalpy, entropy, and free energy are all state functions. There are two main pillars for the conceptual understanding of chemical change, chemical thermodynamics, which is path independent, and chemical kinetics, which describes the factors that determine reaction rate which depend on the path.