Interdisciplinary Note (8 of 12)
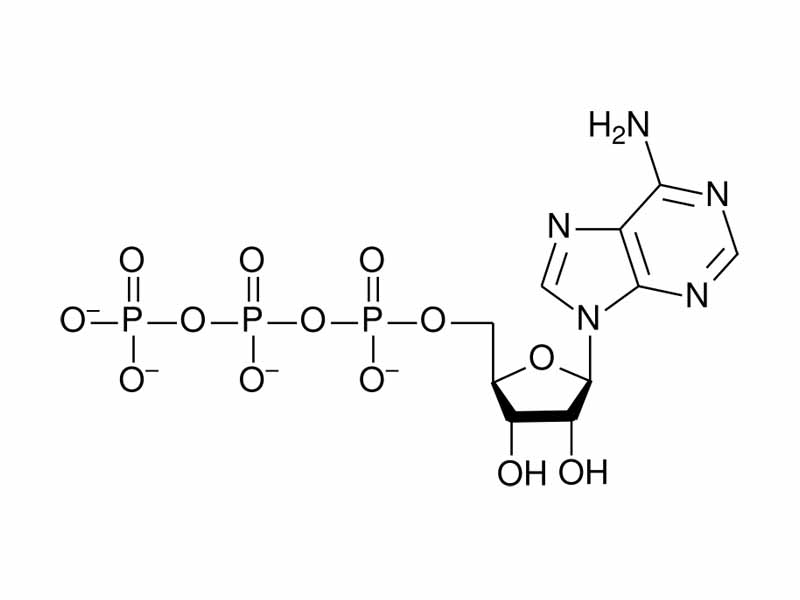
Let us take as a specific example one of the most important biological molecules to discuss internal energy decrease in chemistry, ATP. With ATP hydrolysis, internal energy decrease can largely be explained in the simple terms of electrostatic potential energy. There is more to it (resonance, Mg2+ coordination, solvation), but in a very real sense, ATP is a compressed spring of electrostatic potential energy.
ATP contains a series of three negatively charged phosphate groups. When hydrolysis of ATP occurs, one of the phosphate groups, bearing two negative charges, is allowed to separate from the rest. In ATP the three negatively charged phosphate groups are like a coiled spring. The molecule is not stable thermodynamically but it is kinetically. ATP is stable in solution, its energy harnessed in a kinetic trap. Enzyme catalysis can unlock it, giving the spontaneous hydrolysis of ATP a path to go down. Like heat flow that can only find the cold sink through the engine, ATP can only find the end of hydrolysis through enzymes that couple it to a nonspontaneous process in support of the living system.