Interdisciplinary Note (19 of 29)
Both the IR and 1H NMR spectroscopic analysis of alcohols have some interesting features making spectroscopic analysis of alcohols a very MCAT writer friendly topic. In the infrared spectrum of an alcohol, the O-H stretch is particularly noticeable, appearing as a broad peak in the 3200 to 3650 cm-1 region. The broad peak is due to the fact that variations in the intermolecular associations of the hydrogen bonded alcohol molecules lead to variations in the vibrational frequency of the O-H bond of their hydroxyl group. However, it is possible for the IR spectra of alcohols not to have this broad signal. In a very dilute solution of the sample, or in the gas phase, hydrogen bonding is prevented through lack of proximity. In these cases the broad O-H peak is replaced by a sharp signal.
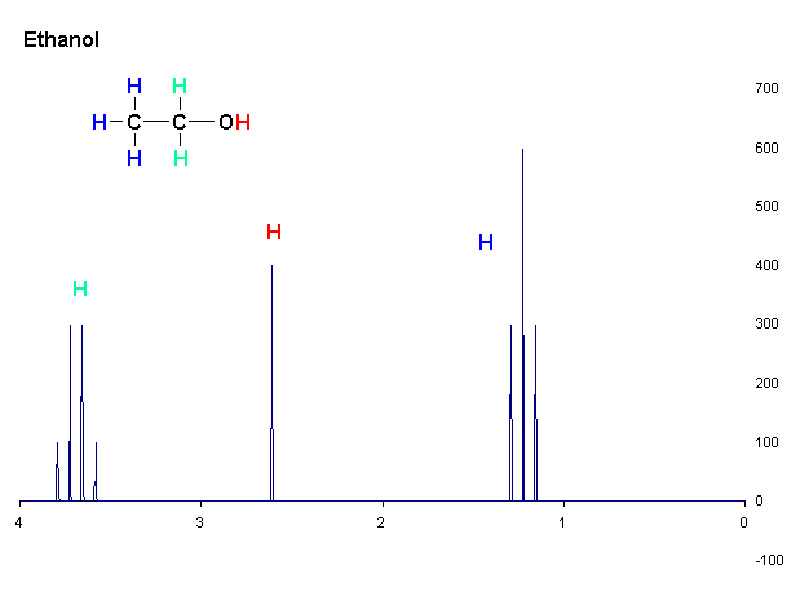
Like IR spectrosophy, the 1H NMR spectroscopy of alcohols has some interesting features. In 1H NMR, the hydroxyl proton appears as a moderately shifted singlet. Because the hydroxyl proton is acidic, however, it does not split the signals of neighbor hydrogens nor is its signal split by them. Furthermore, because the hydroxyl proton is acidic, this singlet will disappear if the alcohol is dissolved in D2O.