Interdisciplinary Note (14 of 16)
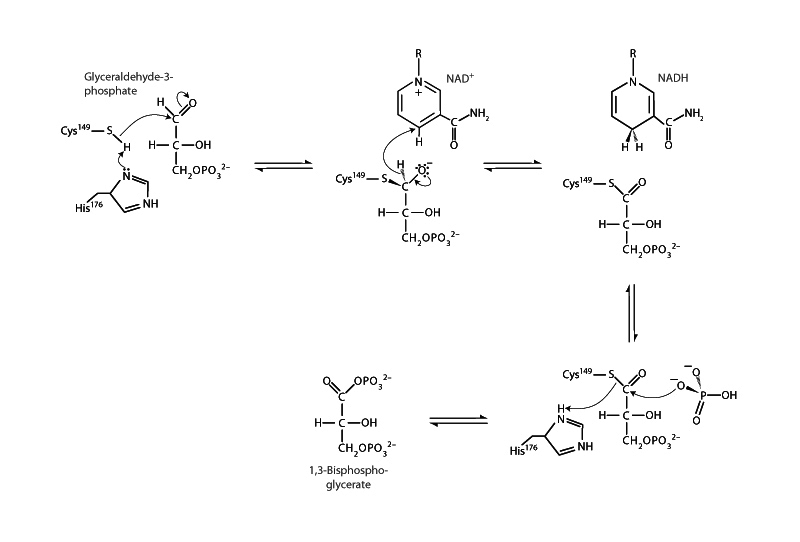
NAD+ has a negative standard reduction potential. Having a negative standard reduction potential means you would have to push electrons uphill from the H2 in a standard hydrogen electrode to get them onto NAD+ and make NADH. How does NAD+ have the redox power to oxidize glyceraldehyde-3-phosphate in the glyceraldehyde-3-phosphate dehydrogenase reaction? The mechanism produces 1,3-bisphosphoglycerate, which is a phosphate anhydride. A phosphate anhydride will already be on the high energy side of carboxylic acid derivatives, but 1,3-BPG is also like a compressed spring of electrostatic repulsion. It would be one thing if the product were a low energy carboxylate, but 1,3-BPG is an activated phosphate anhydride. How does NAD+ do it?
In actuality, if the reaction were taking place with standard concentrations, it would go backwards. The standard free energy change, ΔG0, of the glyceraldehyde-3-phosphate dehydrogenase mechanism is approximately +7 kJ/mol. However, under physiological conditions, the reaction is maintained near equilibrium. There are greater concentrations of reagents than products. Remember that the free energy change does not only depend on how product and reagent compare in themselves - the standard free energy change, but it also depends on what concentrations of reagent and product are actually present in the chemical system.
ΔG = ΔG0 + 2.303 RT log Q
We can say the same thing regarding the cell potential of the reaction using the Nernst equation, which would give us the voltage if we tried to make a battery out of the reaction with the oxidant and reductant located at different electrodes.
E = E0 - [(2.303 RT)/(nF)] log Q
In fact, the cytosol is a fairly oxidizing environment. Although the values can vary with the metabolome of any given cell, the NAD+ to NADH ratio will typically be around 500:1 in the cytosol.