Interdisciplinary Note (12 of 16)
Disproportionation describes a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation state. Biochemists don't generally use the term "disproportionation", however. A biochemist is more likely to call a disproportionation reactions a "dismutation" reaction.
Superoxide, O2-, is a reactive oxygen species (ROS) playing a central roll in physiology. Superoxide can act both as a direct instigator of damage to cellular components and as the precursor of other damaging ROS. To initiate the pathway of neutralizing superoxide, the enzyme superoxide dismutase (SOD) carries out the following disproportionation reaction.

Depending on which SOD isoenzyme is involved, the SOD reactive center employs either coordinated zinc ion or manganese ion in a multistep process. In the equations below M = Cu (n = 1) or M = Mn (n = 2).

The hydrogen peroxide produced through the activity of superoxide dismutase itself is a very damaging ROS. Catalase is a very important enzyme for protecting the cell from damage by hydrogen peroxide.

When you scanned the catalase reaction equation above, did you notice that it is also a disproportionation reaction? It will really help you on the MCAT to internalize the skill of picking up on the changes in oxidation state quickly, as a natural part of "clearing" the reaction mentally, recognizing a redox reaction and then analyzing what's going on in redox terms as a natural part of reading the reaction.
One of the most interesting and salient aspects of catalase is that the enzyme has attained enzymatic perfection. In other words, it's catalytic efficiency (kcat/KM) approaches the upper boundary where the only factor limiting rate is diffusion. As soon as catalase and H2O2 bump into each other the substrate binds; doesn't come unbound; and then turns over immediately. One catalase molecule can convert millions of hydrogen peroxide molecules to water and oxygen each second.
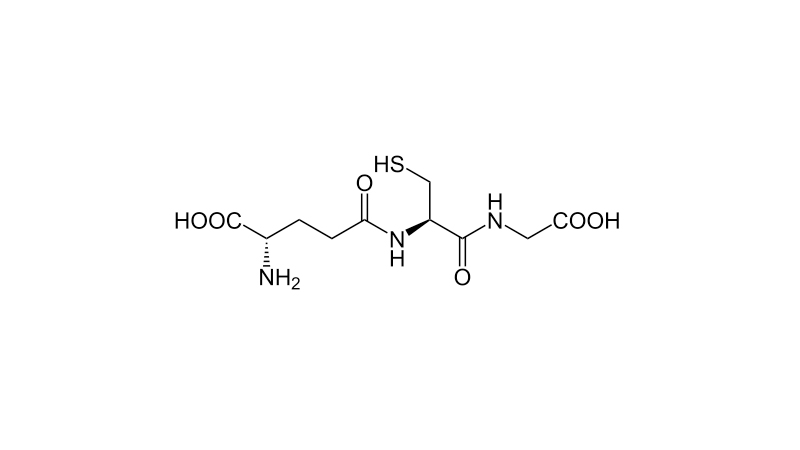
Catalase is not the only important enzyme for neutralizing peroxide. Another important enzyme to deal with hydrogen peroxide is glutathione peroxidase, although it does not carry out disproportionation, but simple reduction, and not only hydrogen peroxide, but also lipid hydroperoxides. Free radicals produced through a variety of misfiring enzymes as well as ionizing radiation initiate a chain reaction that eventuates on the membrane as a reactive peroxy group on a lipid, a lipid hydroperoxide. Glutathione is the reducing agent in the reaction. Glutathione is built of three amino acids, glycine, cysteine, and glutamate, though it's a little different than a simple tripeptide, because the glutamate is attached to cysteine through its side-chain carboxyl group instead of its α-carboxyl group. The reactive center is the thiol group of cysteine. Glutathione has a reduced form and an oxidized form. The oxidized form is glutathione disulfide. Together, glutathione and glutathione disulfide, comprise the two sides of the most important redox buffer in physiology.
When there is a low pH, it's like the aqueous environment has a proton pressure. A basic environment is like a proton suction. Similar to this line of thinking, the environment might have an electron pressure. This is a reducing environment, or there could be an electron pull. It could be an oxidizing environment. Just like an acid-base buffer maintains the pH within a narrow range, a redox buffer balances the redox environment.
Glutathione peroxidase reduces peroxides. In the process, glutathione is oxidized to its disulfide form. Just like the equilibrium of a buffer absorbs a strong acid, forming a weak acid, the glutathione equilibrium converts a strong oxidizer into a weak oxidizer, glutathione disulfide.

The redox buffer will then be recharged by NADPH through glutathione reductase.

To provide reducing equivalents to maintain the balance of glutathione vs. glutathione disulfide and thus to serve in the battle against ROS is one of the primary functions of NADPH, and by extension, the pentose phosphate pathway (to provide reducing equivalents and ribose to bioynthesis are the other main functions).
A big impetus with these notes is to discuss some of the themes that are uppermost in the minds of AAMC in constructing MCAT passages. Often these themes are at the edge of expected foreknowledge for the exam, where it is a figure of merit to have your footing. Disproportionation, ROS, enzymatic perfection, glutatione. These are all themes that epitomize that aspect of the exam.