Interdisciplinary Note (9 of 9)
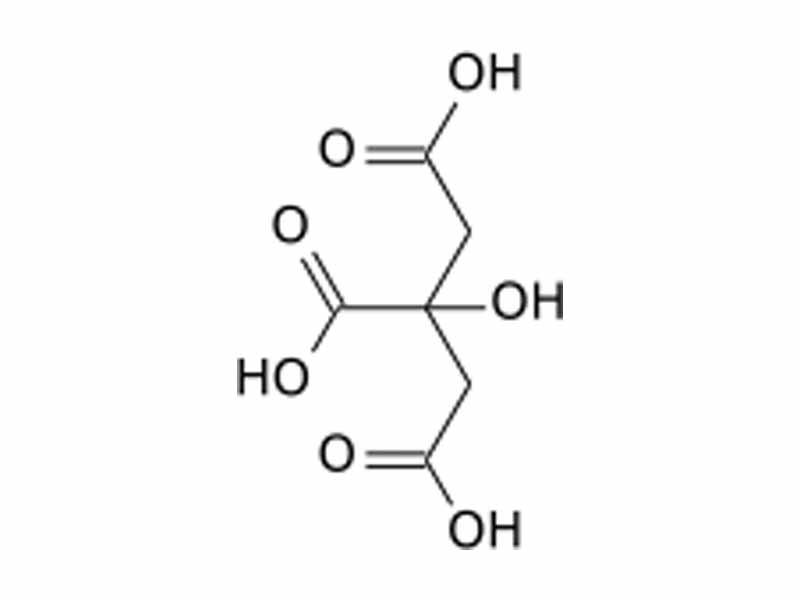
When Hans Krebs first proposed the citric acid cycle, there was resistance to acceptance for a few years because evidence seemed to contradict it. For example, if you input 14C labeled acetyl CoA into the cycle, the oxaloacetate formed at the end after one cycle will always have those 14C atoms. The 14C will not be detected at all in the CO2 released by the isocitrate dehydrogenase and α-ketoglutarate dehydrogenase steps. This seemed impossible to many scientists at the time because citrate is achiral. The two ends of citric acid should be chemically equivalent.
The citrate molecule is symmetric around its central carbon. Therefore, according to dogma from bench-top organic chemistry, it should be impossible for a reaction to distinguish the two ends of citrate chemically. In normal bench-top chemistry, it is a dogma that you cannot form a pure optical isomer from an achiral precursor. The citric acid cycle provides us with a quintessential demonstration, however, of the capability of an enzymatic reaction to form a pure optical isomer from an achiral precursor.
Picture the citrate molecule in space. To the left is the carboxyl group on the central carbon. To the right is the hydroxyl group. Above and below are the two acetyl groups. The central carbon is what is known as "prochiral", ie. one step from chiral. The molecule is tetrahedral around the central carbon, of course, so you could picture the carboxyl and hydroxyl angled towards you on the left and right and the two acetyl groups angled away top and bottom, as in a Fischer projection. As expected with any enzyme, the aconitase active site has a complex, asymmetric, three dimensional shape. As chirality is defined in the abstract, the active site is chiral. It would not be superimposable on its own mirror image. Let us suppose that the domain for binding the carboxyl group were on our left rear and the domain for binding hydroxyl were on the right rear, and the substrate cannot rotate. If the catalytic residues were towards the top angled away from our perspective in the active site, it would always be towards that end that the reaction would begin. Because the active site is itself asymmetric, it can distinguish between the two seemingly equivalent substituents on the prochiral carbon. (Aconitase is actually more complicated than this. The intermediate, aconitate, actually flips after elimination and before rehydration).
The capability of enzymes to create pure optical isomers from achiral precursors is absolutely fundamental to biochemistry. The general chemistry principle is that this is possible on the bench-top with an asymmetric catalyst with multiple points of attachment. It's a focus in bench-top chemistry to discover such catalysts. In biochemistry, this degree of stereospecificity is the norm. When transaminase transforms achiral pyruvate into alanine, it will always form only L-alanine. When yeast alcohol dehydrogenase delivers a 2H hydride from NADH to acetaldehyde, the location of the deuterium is always be stereospecific in ethanol. There are myriad examples.