Interdisciplinary Note (8 of 9)
Two of the enzymatic steps in β-oxidation are, respectively, examples of organic chemistry reactions that are not on the AAMC topic outline list of organic reactions. However, judging from their published materials, AAMC seems to have fondness for these two reactions anyway.
The addition of water in the enoyl-CoA hydratase step is a Michael addition. Enoyl-CoA is an α,β-unsaturated carbonyl compound, ie. it has a double bond between one and two steps away from a carbonyl group. Michael addition describes a reaction in which a nucleophile adds at the β position. The addition of a nucleophile at the β position of an α,β-unsaturated carbonyl compound is favorable because the π electrons in the double bond can move onto the α carbon and they will be in resonance within an enolate. The MCAT probably would not be super aggressive about Michael addition, but it seems like a good thing to know about.
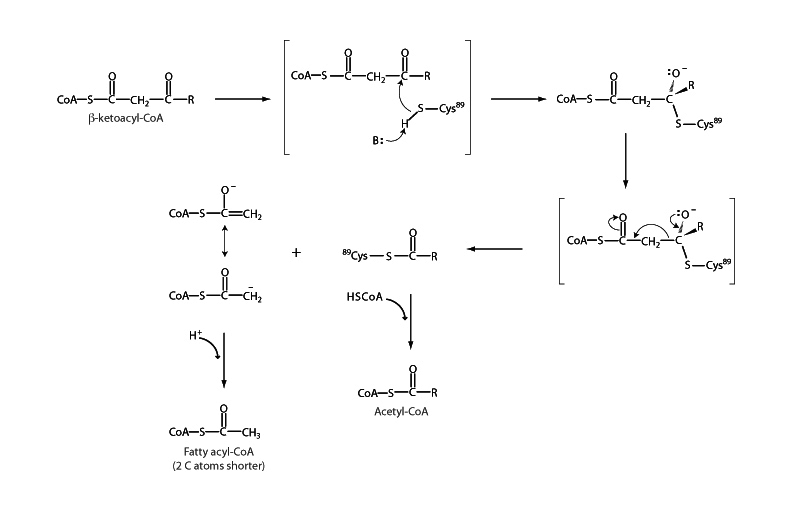
The β-ketoacyl-CoA thiolase step, where cleavage occurs to liberate acetyl CoA, is a retro-Claisin condensation (Claisin condensation in reverse). Claisin condensation is the carboxylic acid world version of aldol addition from aldehyde & ketone chemistry. However, instead of the enolate of one carbonyl compound adding to another, it's an enolate ester adding to an ester and the reaction then continues with collapse of the tetrahedral product of addition, in other words, nucleophilic acyl substitution. The thiolase step is Claisin condensation happening backwards. Likewise, the exam is probably not going to be brutal about Claisin condensation, but it seems like a good thing to know about.