Interdisciplinary Note (8 of 20)
One of the themes of the first part of this MCAT course will be to develop our idea of the internal energy of chemical substances building from the fundamental tools of mechanics and basic electricity & magnetism. We will return to this theme from many directions. Classical physics is extremely helpful to the conceptual understanding of chemistry, but you must always bear in mind the departures from classical ideas in quantum mechanics. We will devote a great deal of effort to developing the ability to conceptualize chemical change in terms of the main forms of energy from physics which are relevant.
The two main forms of energy relevant to chemical transformations are the electrostatic potential energy among the particles and kinetic energy of the particles. In fact, for an ideal gas, translational kinetic energy of the particles is the only form of internal energy. Translational kinetic energy is kinetic energy of a particle whose center of mass is moving through space. In a real substance kinetic energy can also exist as rotational kinetic energy and vibrational kinetic energy in addition to translational kinetic energy. In statistical aggregate, we refer to the kinetic energy at the particle level as the thermal energy.
In addition to the thermal energy a substance possesses, within a real substance, not an ideal gas, you have charged particles, the protons in the nucleus, the electrons in orbit around the atoms. Electrostatic force interactions occur among these particles, holding electrons in atoms and holding atoms in covalent bonds. As proximity relationships change through a chemical transformation, the electrostatic potential energy states within the matter are changing. As a general rule, when opposite charges move apart, electrostatic potential energy is increasing. Think about how if you were doing this, it would require the input of mechanical work on your part. Likewise, if like charges are forced together, electrostatic potential energy increases. At the thermodynamic level, this translates to an internal energy increase. This is from the thermodynamic system perspective, which looks at a mole of substance as a statistical aggregate. When opposite charges move more closely together in a reaction, such as when oxygen gains control over electrons in a reaction and draws them in closely to its powerful nucleus, electrostatic potential energy decreases. Imagine how it would take mechanical work for you to move the electrons the other way. Likewise, when like charges move further apart, electrostatic potential energy decreases, like a spring letting go.
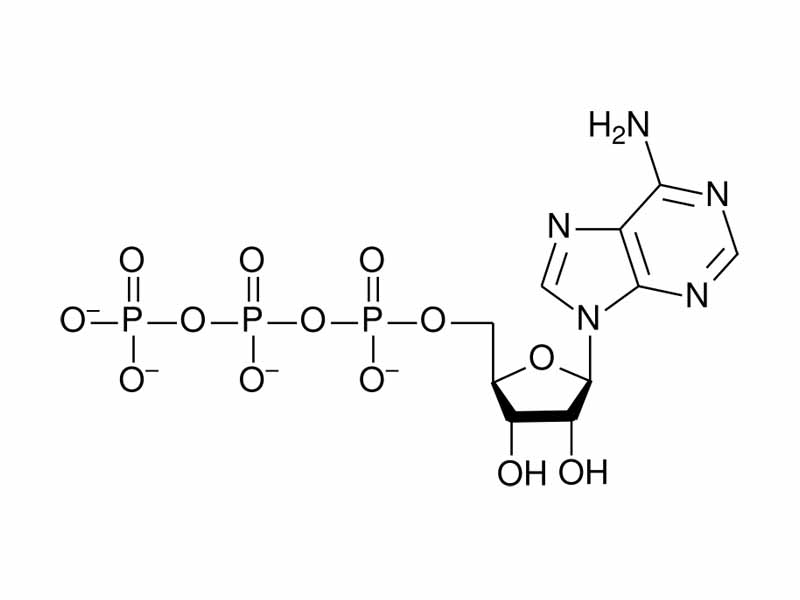
For example, take a look at the structure of ATP in the figure. Notice the three phosphate groups, the α, β, and γ phosphates in a row. From the simplistic perspective of physics, you have three negative charge densities packed close together in the triphosphate portion of ATP. From an electrostatic potential energy perspective, ATP is like a compressed spring. Hydrolysis of ATP will allow the γ phosphate to separate from the rest of the molecule. This represents an internal energy decrease of a mole of ATP because it is an electrostatic potential energy decrease for a molecule of ATP. (There's more to it than the mutual repulsion of the phosphates. Of course, there's always more to it. A greater degree of resonance stabilization of the products of hydrolysis, for example, solvent effects, and the role of magnesium ions, but you won't go wrong thinking of ATP like a compressed spring. This is where its phosphoryl transfer potential comes from.)