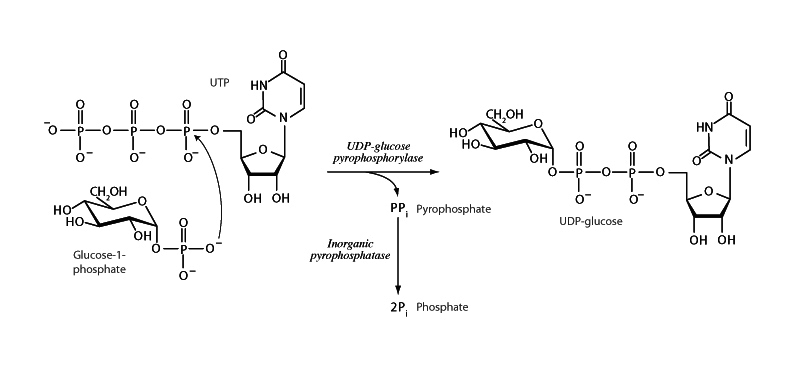
Uridine diphosphate glucose (UDP-glucose) is an activated form of glucose. UDP is a better leaving group than the C-1 hydroxyl of glucopyranose. UDP-glucose typically serves as the substrate of glycosyltransferases such as glycogen synthase.
UDP-glucose pyrophosphorylase catalyzes the reaction of UTP and glucose-1-phosphate. A phosphoryl oxygen of glucose-1-phosphate attacks the α phosphorus of UTP along the in-line phosphoryl transfer pathway to form a new phosphoanhydride linkage while releasing pyrophosphate. Because this reaction is merely a phosphoanhydride exchange, there is not much to push this reaction forward thermodynamically. However, the pyrophosphate formed will be subsequently cleaved by the omnipresent enzyme, inorganic pyrophosphatase. Pyrophosphate cleavage is a highly exergonic process. Subsequent breakdown of pyrophosphate drives the reactions forward involving alpha-beta cleavage of nucleoside triphosphates.