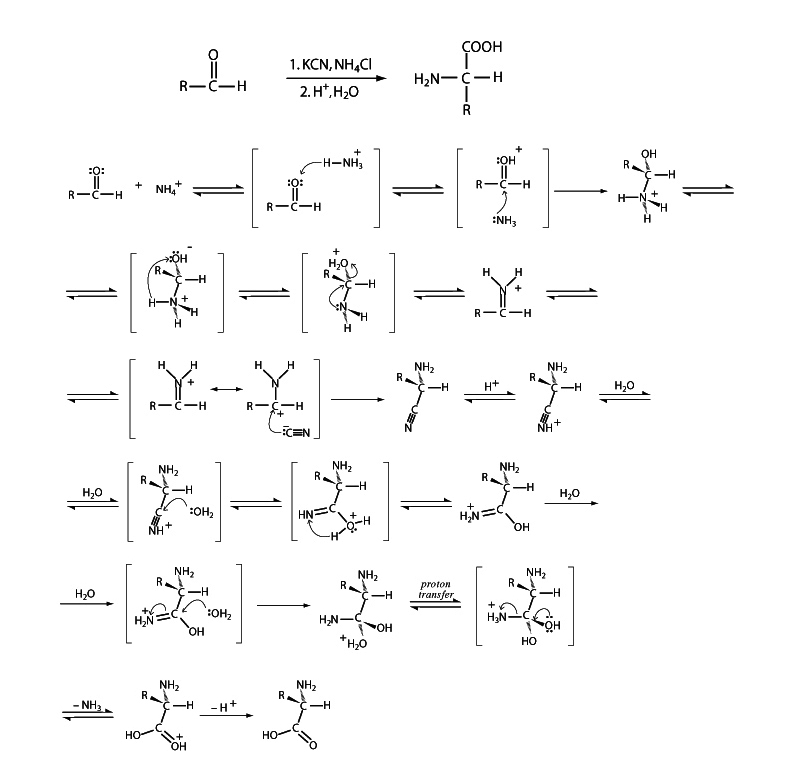
The Strecker amino acid synthesis employs an aldehyde substrate for the synthesis of an α-amino acids. Ammonia serves as the amine precursor in the synthesis and cyanide as the carboxyl precursor. The first step involves nucleophilic addition of ammonia to the aldehyde to form an imine. Next, reaction of the imine with a cyanide nucleophile is carried out to form an α-aminonitrile. Subsequent hydrolysis of the nitrile group of the α-aminonitrile transforms it into the carboxyl group of the finished α-amino acid.The traditional Strecker synthesis yields a racemic mix-ture of L- and D- amino acids, but several procedures utilizing asymmetric catalysts have been developed.