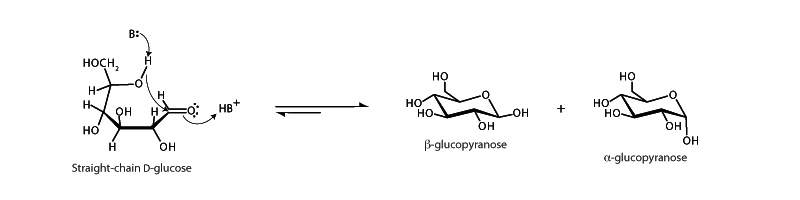
The open form of D-glucose, like many sugars, can cyclize in aqueous solution to form a hemiacetal. This reaction is an example of hemiacetal phase of acetal formation in which an equivalent of alcohol forms a tetrahedral intermediate with an aldehyde. In glucose ring formation the nucleophilic hydroxyl group approaches the anomeric carbonyl from either below or above the plane of the forming ring, so two stereoisomers are possible, either β-D-glucopyranose or α-D-glucopyranose. β-D-glucopyranose is somewhat more stable. The α and β forms of D-glucose interconvert in aqueous solution, by a process called mutarotation. The equilibrium mixture consists of about one-third α-D-glucose and two-thirds β-D-glucose, along with very small amounts of the linear form.