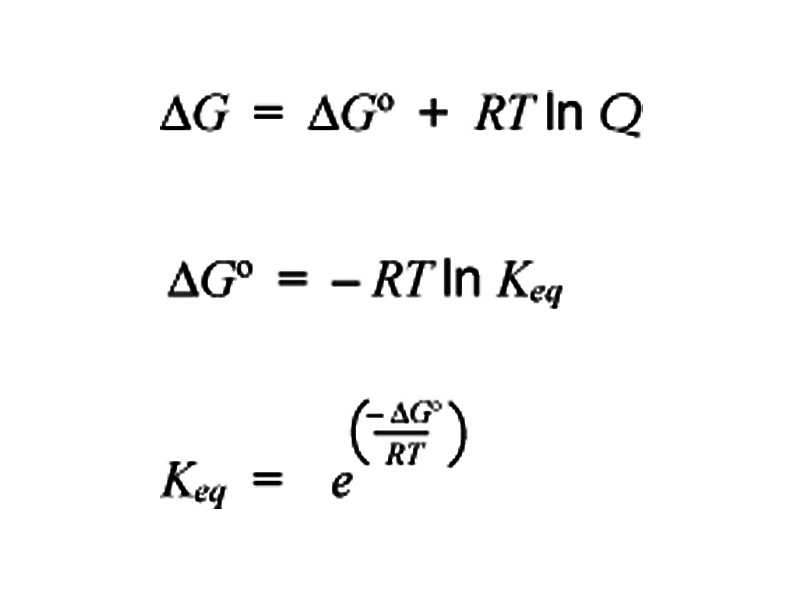In our earlier discussion of Thermochemistry, we reviewed the concepts of internal energy change and enthalpy change. This equipped us to describe the changes in a substance as a thermochemical system. We learned how to compare the products and reagents in a chemical reaction in terms of the allotment of energy between the system and its surroundings. If the system completely changed from Reagents A to Products B, does heat flow in or out? At this stage, we are ready to seek to understand spontaneity in chemical transformations. What is the availability of energy in a chemical system for drive a reaction forward? We are describing the free energy in a chemical system, energy that when it is expended during chemical tranformation increases the entropy of the universe. Free energy is expended until the equilibrium state for the system is achieved. At equilibrium, heat flows between the system and its surroundings become microscopically reversible. The forward direction is just as likely as the reverse because the entropy associated with heat flows in either direction is the same.
On the MCAT, you probably will not have too many direct chemical thermodynamics questions, but the topic is the scaffolding on which much else is built. I believe chemical thermodynamics to be one of the most important subject areas in science. Most students find chemical thermodynamics to be a very abstract subject. This is unfortunate. I think this happens because of the way that chemical thermodynamics is approached in undergraduate general chemistry, without physics underneath and biology above. However, if you approach chemical thermodynamics with a concrete basis in force and energy, you can relate the internal energy changes driving heat flow and entropy change to a fundamental understanding of the particle level in substances, and when you bring chemical thermodynamics to your understanding of biochemistry, your appreciation of life processes will become much more coherent and much more interesting.
WikiPremed Resources
Chemical Thermodynamics & Equilibrium Practice Items
Conceptual Vocabulary Self-Test
Basic Terms Crossword Puzzle
Basic Puzzle Solution
Conceptual Vocabulary for Chemical Thermodynamics
Chemical Thermodynamics
The equilibrium constant is the reaction quotient describing the state in which the chemical activities or concentrations of the reactants and products have no net change over time.
A spontaneous process is a chemical reaction in which a system releases free energy and moves to a lower, more thermodynamically stable, energy state.
Chemical thermodynamics is the mathematical study of the interrelation of heat and work with chemical reactions or with a physical change of state within the confines of the laws of thermodynamics.
The term thermodynamic free energy is a measure of the amount of work that can be extracted from a system.
An endergonic reaction (also called an unfavorable reaction or a nonspontaneous reaction) is a chemical reaction in which the standard change in free energy is positive.
An exergonic reaction is a chemical reaction where the variation of Gibbs free energy is negative.
Le Chatelier's principle states that if a chemical system at equilibrium experiences a change in concentration, temperature, volume, or total pressure, the equilibrium will shift in order to partially counter-act the imposed change.
The Gibbs free energy is a thermodynamic potential which measures the useful or process-initiating work obtainable from an isothermal, isobaric thermodynamic system.
A dynamic equilibrium occurs when two reversible processes proceed at the same rate.
The reaction quotient is a quantitative measure of the extent of reaction, the relative proportion of products and reactants present in the reaction mixture at some instant of time.
The entropy of mixing is the change in the entropy when two different chemical substances or components are mixed.
Activity in chemistry is a measure of how different molecules in a non-ideal gas or solution interact with each other, extending the idea of concentration to more complex systems.
An activity coefficient is a factor used in thermodynamics to account for deviations from ideal behaviour in a mixture of chemical substances.
Thermodynamic potentials are parameters associated with a thermodynamic system and have the dimensions of energy which describe the capacity for spontaneous change in a thermodynamic system when it is subjected to certain constraints.
The chemical potential of a thermodynamic system is the amount by which the energy of the system would change if an additional particle were introduced, with the entropy and volume held fixed.
Fugacity is a measure of chemical potential in the form of 'adjusted pressure' directly related to the tendency of a substance to prefer one phase over another.
The thermodynamic limit is reached as the number of particles in a system approaches infinity and the thermodynamic behavior of a system is asymptotically approximated by the results of statistical mechanics.
The Helmholtz free energy is a thermodynamic potential which measures the useful work obtainable from a closed thermodynamic system at a constant temperature.
The Gibbs paradox, also known as mixing paradox, involves the discontinuous nature of the entropy of mixing.
The Gibbs' phase rule relates the number of phases present in equilibrium, the number of degrees of freedom, pressure and composition of the phases present, and the number of chemical components required to describe the system.
Non-equilibrium thermodynamics is a branch of thermodynamics concerned with studying time-dependent thermodynamic systems, irreversible transformations and open systems.
A dissipative system is a thermodynamically open system which is operating far from thermodynamic equilibrium in an environment with which it exchanges energy, matter and/or entropy.
The Debye-Hückel limiting law provides a method for obtaining activity coefficients for solutions that contain ionic solutes.
The Van't Hoff equation in chemical thermodynamics relates the change in temperature to the change in the equilibrium constant given the enthalpy change.
The Gibbs-Duhem equation in thermodynamics describes the relationship between changes in chemical potential for components in a thermodynamical system, showing that in thermodynamics intensive properties are not independent but related.