Interdisciplinary Note (12 of 22)
It's easy to become accustomed to thinking only about whether or not the bonds within a molecule are polar in deciding the intermolecular force relationships. Typically, you examine the electronegativity differences between bonded atoms and assign the polarity of the bonds and go from there to decide whether the molecule is nonpolar or polar.
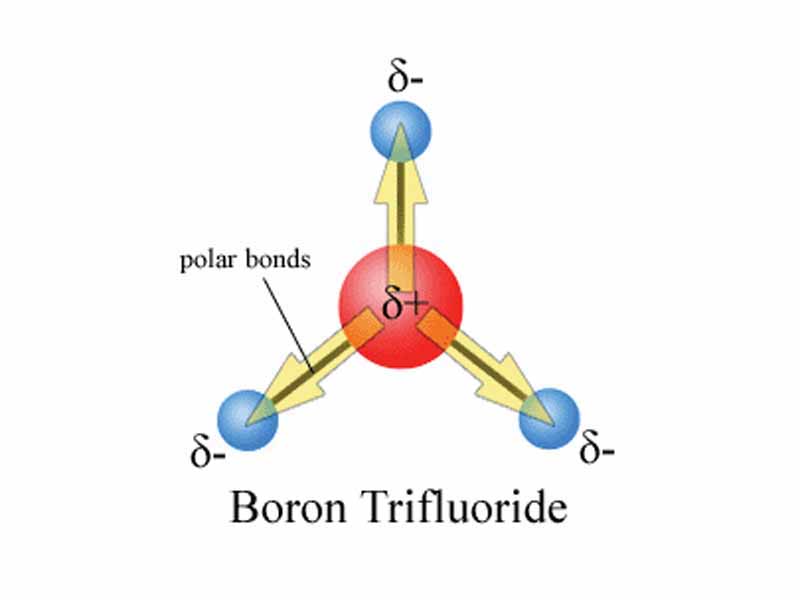
However, the polarity of bonded atoms is not the only factor in determining the overall polarity of a molecule, and by extension, intermolecular force. Molecular geometr is also important.
For example, if the various dipole moments within a molecule are oppositely directed in space, even though the particular bonds are polar, the molecule may have no net dipole moment at all, in which case the degree of intermolecular force is weak. Carbon dioxide is the archetypal example, a linear molecule in which the dipole moments of the carbon-oxygen bonds are oppositely directed.
From a test-writer's perspective, this kind of exception makes a good multiple choice question. Keep VSEPR in mind on the MCAT if you are faced with interpreting the overall polarity of a molecule. Don't forget to ask yourself whether the dipole moments of individual bonds cancel each other out.