Interdisciplinary Note (16 of 20)
The relationship of a transformation with regard to a particular kind of statistical view of order will be a crucial point of view we will learn later to apply to judge the transformations of energy. When you are applying this point of view, you are judging a transformation in terms of the total change in entropy (ΔS) of the system plus the change in entropy of surroundings due to heat flow, which gives us the change in entropy of the universe. (Entropy is an idea that takes a bit of rumination.) This is the Gibbs free energy way of looking at things, an incredibly useful framework for viewing physical change because it helps you to understand why some directions of chemical change are spontaneous and some are not.
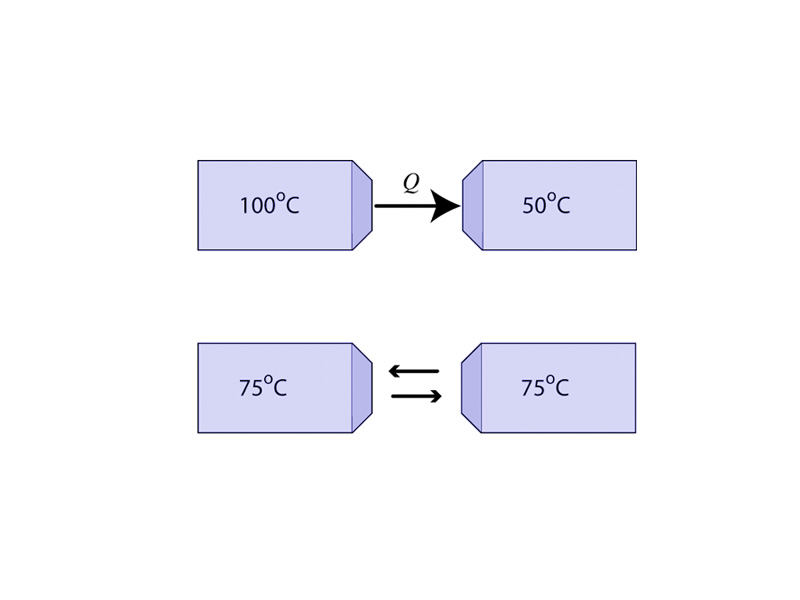
We will have a great deal more to say about this as this course progresses, but here, in the context of energy as a topic of mechanics, let it suffice to understand that some kinds of energy represent a more or less disordered state than others. Spontaneous change leads to an increase in the disorder (entropy) of the universe. This is the 2nd law of thermodynamics. You drop a book on the table. Potential energy become kinetic energy, and then through the collision, the kinetic energy became vibrations and thermal energy in the table and sound. The total energy was conserved, but the thermal energy and sound are not going to return and lift the book back up to your hand. It doesn't happen for the same reason a deck of cards doesn't unshuffle itself.
Most real world transformations are spontaneous, i.e. irreversible. In other words, it is much more likely for the sliding block to produce sound than for sound to make the block slide back up the plane.
The understanding that the entropy of the universe is always increasing is a very important concept governing the transformations of physical systems within their surroundings. When you move further into the physical sciences, you want to add to the first question: What is happening with energy? A second question: What is happening with entropy? Could this change reverse itself on its own?
This is a preview of a very important set of concepts which we will be a steadily increasing focus of this course. For now, keep in mind that not all directions of change are equal. Real world energy transformations are not reversible.