Interdisciplinary Note (11 of 11)
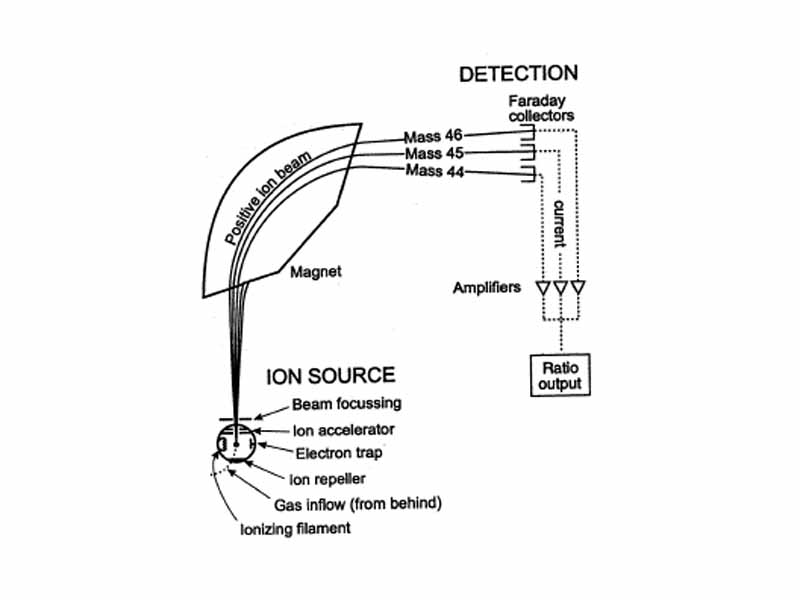
After fragmentation of the molecule in a mass spectrometer, cations with the lowest charge to mass ratio are deflected least by the magnetic field, while those with the highest charge to mass ratio are deflected most. The instrument scans over the range of charge to mass ratio, the mass spectrum, varying either the initial particle acceleration or the magnetic field to cause a particular charge to mass ratio to be focused onto the detector.
The most intense peak is the base peak. The base peak is not always the molecular ion peak because some compounds are very prone to fragmentation. This is especially true when fragments can be formed that are especially stable carbocations, such as the benzyl cation.
Small peaks one or two units adjacent to a large peak correspond to the presence of isotopes of elements present. Isotopic clusters are especially prominent in substances containing chlorine or bromine because of the high relative abundance of these isotopes.