Interdisciplinary Note (10 of 11)
Ultraviolet Light corresponds to the portion of the electromagnetic spectrum with wavelength just shorter (higher frequency) than visible light. The visible region extends from approximately 800 to 400 nm and UV light from 400 nm to 200 nm.
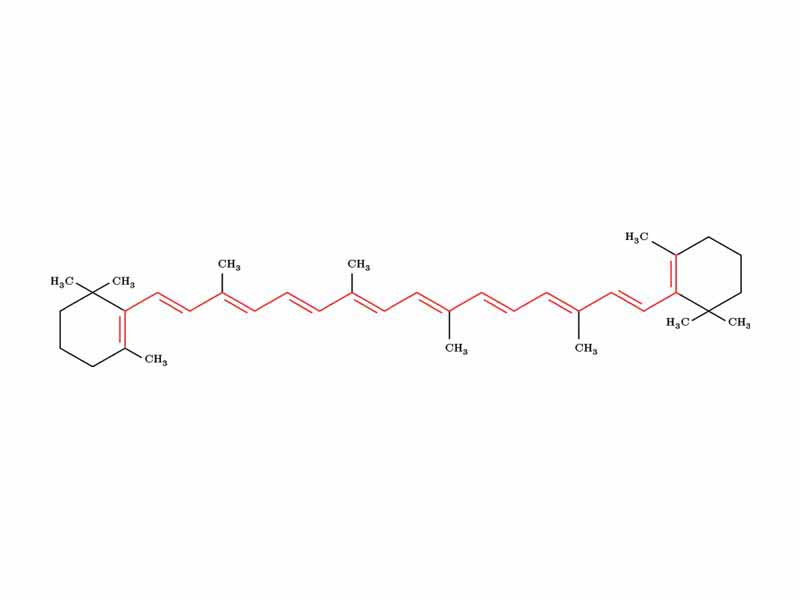
UV spectroscopy is most useful when conjugated systems are present. The more extended the conjugation, the smaller the HOMO-LUMO energy gap (HOMO = Highest Occupied Molecular Orbital. LUMO = Lowest Unoccupied Molecular Orbital). The most extended conjugated systems are pigments that have absorption maxima in the visible range, not the UV range, although lmax may be in the UV range, the compound can still be colored with a broad peak that extends into the visible range.