Interdisciplinary Note (10 of 22)
One very important MCAT context for orbital theory is in understanding the hybrid orbitals of organic chemistry, their spatial geometry and energy characteristics. In forming chemical bonds, atomic orbitals undergo both inter- and intra-molecular orbital hybridization.
Intermolecular orbital hybridization occurs between orbitals of different atoms.
Intramolecular orbital hybridization occurs between orbitals of the same atom.
In organic chemistry, intra-molecular orbital hybridization (sp3, sp2, etc.) plays a roll in determining the structural and chemical properties of the functional groups.
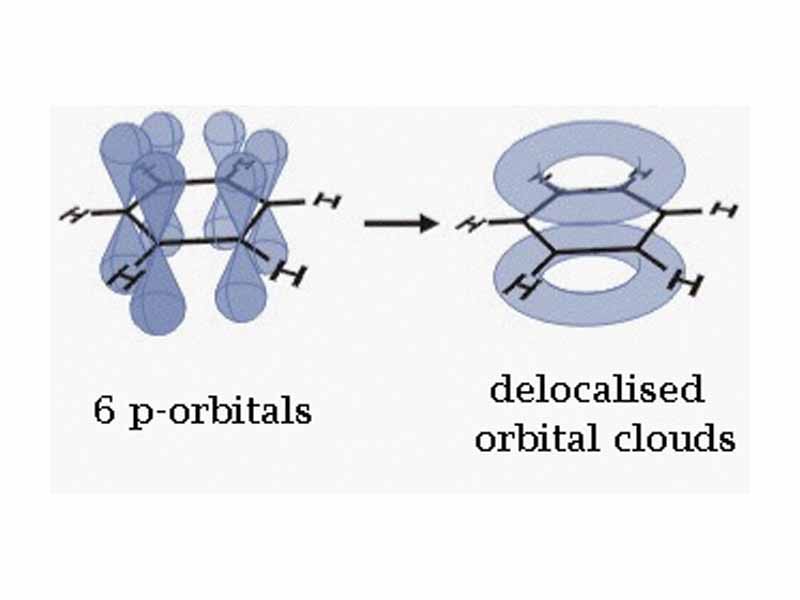
The π orbitals of an extended sequence of sp2 hybridized atoms may combine to form a π system, wherein electrons are delocalized through orbital hybridization over a large number of atoms. Such resonant forms are lower energy, i.e. more stable than non-conjugated systems.
Why is that so. A simplistic way of looking at it is to say that extended conjugation allows electron charge to spread out and decrease in potential energy, a useful way of looking at it.