Interdisciplinary Note (5 of 22)
The electronegativity of an element is a transcendentally helpful thumbnail signifier of its chemical 'personality'. Along with the valence, or outer shell electron configuration, the electronegativity of an element tells you a great deal about how it will behave in many contexts. The greater the electronegativity of an element, the stronger its pull on the electrons it shares with other atoms in chemical bonds. When you walk into the MCAT, you want to be in the habit of an automatic consciousness of the electronegativies of the atoms at play in any chemical situation. This will make chemistry more coherent and intuitive.
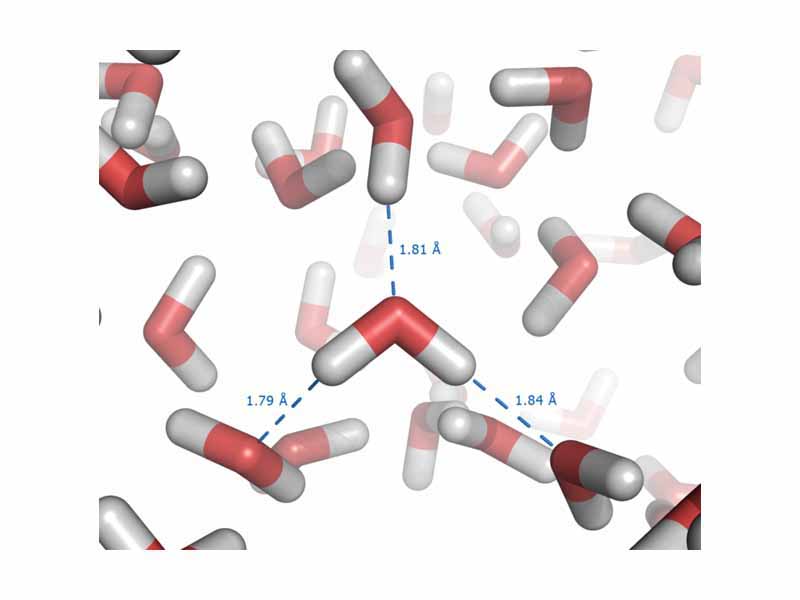
One very significant role for electronegativity is in determining the polarity of covalent bonds, which then determines the degree of intermolecular force. The greater the electronegativity difference between the bonded atoms, the more polar the bond. The shared electrons spend more time around the more electronegative atom, giving it a partial negative charge and leaving the other atom partially positive. The bonded atoms form a permanent dipole. Furthermore, because there are discrete positive and negative charge densities, the dipole can be attracted by other dipoles leading to strong intermolecular forces.
This is especially true in the case of one subtype of polar bonds, which are strong enough to earn their own name, the hydrogen bond. Hydrogen bonds are intermolecular forces that arise in substances where hydrogen is bound to a polar element, such as oxygen, and the positive pole is a bare hydrogen nucleus. Especially strong interactions result.