Interdisciplinary Note (2 of 26)
In kinetic theory we approached temperature as a measure of the average kinetic energy per particle in an ideal gas. However, substances whose particles have many ways to vibrate or rotate have more places to put the kinetic energy than just moving in a line through space. Think about how many ways a particle has to move, translational, vibrational, and rotational, and think about how much energy there is on average in each one, in each degree of freedom. That is what temperature measures. Because not all substances have the same number of ways to move at the particle level, substances may have different molar heat capacities, in other words. That is the kinetic theory approach to temperature which we have been using very fruitfully thus far.
However, now that we are moving into the second law of thermodynamics, let us expand our conception of the temperature. Let us begin to look at temperature within a different conceptual framework and start thinking of temperature as a sort of potential function for the escaping tendency of heat.
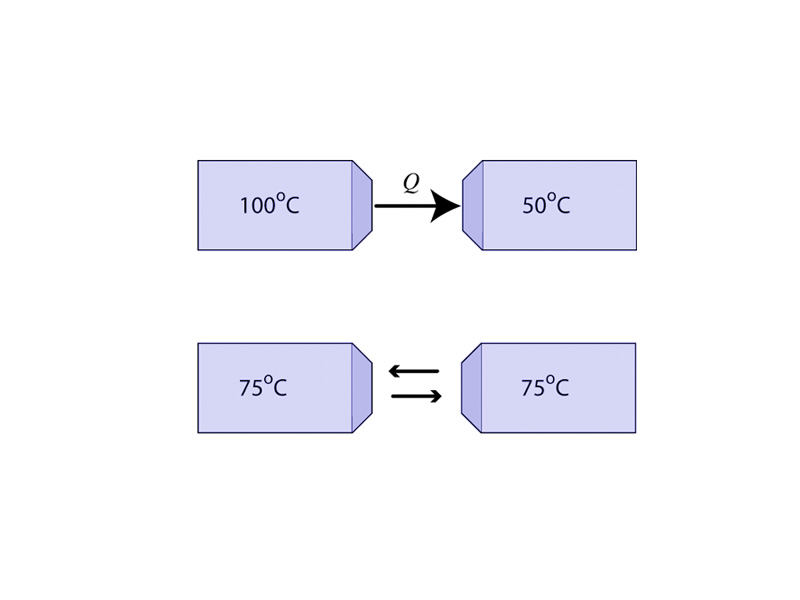
If the temperature is high in a region, it will probably not be so high for very long. As heat flows from the warmer to the cooler object in thermal contact, the increase in entropy in the cold object is greater than the decrease in entropy for the warm object. Think about that! The heat brings more disorder to the cold object than is lost by the hot object. The total entropy in the universe increases. The temperature difference predicts the direction of spontaneous heat flow.
Let's look at this with a little math. When heat flows from the warm object, it loses an amount of entropy equal to Q/Th (This is a simplification because temperature is changing),and the cold object gains an amount of entropy equal to Q/Tc. Th is greater than Tc, so Q/Th must be less than Tc until the two objects are in equilibrium at the same temperature. Therefore, heat flowing from a hot object to a cold one increases the disorder of the universe. The entropy gained by the cold object is greater than the entropy lost by the warm object. The entropy of the universe is increasing. The heat flow is spontaneous.