Interdisciplinary Note (11 of 12)
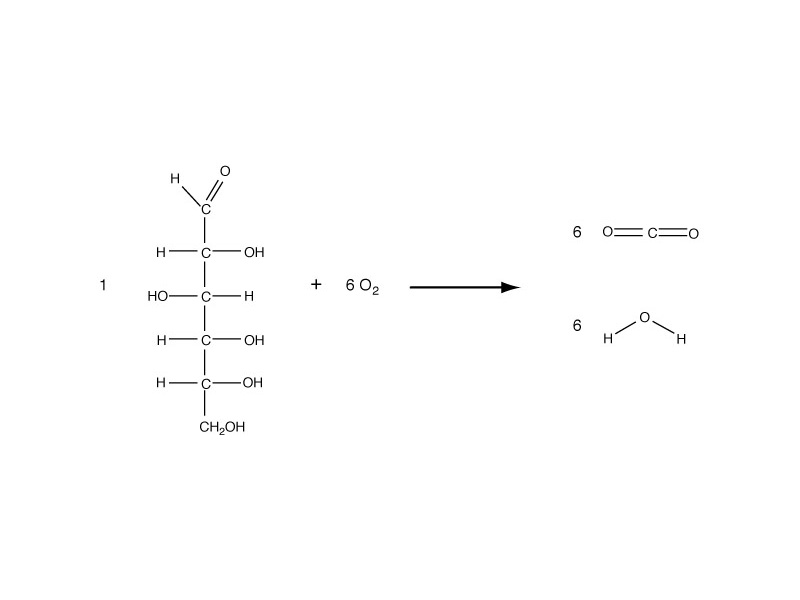
Hess's Law of Heat Summation is a correlative of the more general proposition that changes in the thermodynamic functions describing a chemical system do not depend on the reaction pathway but on the initial and final states of the system. This is very helpful for understanding the changes of oxidative metabolism, which are extremely complex in path, but simple stoichiometrically.
The oxidation of nutrient molecules (glucose, triglyceride, etc) to CO2 and H2O produces the same internal energy change, enthalpy change, or free energy change whether by combustion in the laboratory or through the metabolic pathway.
An important related proposition is that enzymes accelerate the rate of a chemical reaction but do not change the position of equilibrium. A catalyst can change the reaction pathway but not the overall thermodynamics. While the kinetics of a reaction depend on the path, the chemical thermodynamics do not.
Almost every MCAT will have some question to see if you can comfortably separate kinetic (path dependent) reasoning from thermodynamic (path independent) reasoning.