Interdisciplinary Note (1 of 17)
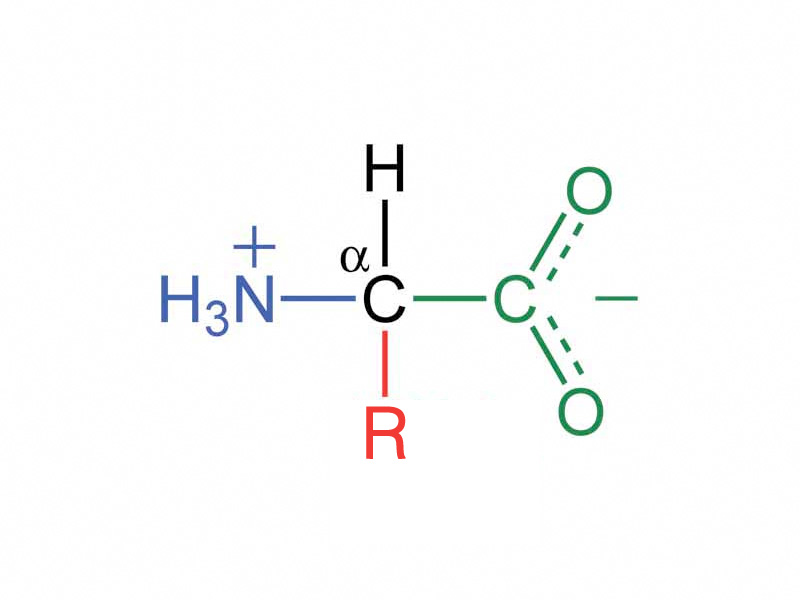
α amino acids have a carboxyl group, amine group, hydrogen and a side chain bound to a central carbon (in proline, the amine group and the side chain have also formed a ring).
Except for glycine which is achiral, the α carbon is a chiral center whose configuration is normally specified using the D,L notational system. Amino acids from proteins in nature have the L configuration at their α carbon. D and L symbols are associated with absolute configuration based on the dextrotatory and levorotatory forms of glyceraldehyde. The designation does not mean that the amino acid is itself dextrarotatory or levarotatory.
α amino acids can be categorized based on whether their side chain is nonpolar, polar (but nonionizable), acidic or basic. Because there is both an amine group and a carboxyl group bound to the central carbon, amino acids are amphoteric. The isoelectric point is the pH at which an amino acid bears no net charge, corresponding to the pH of maximum zwitterion concentration. For amino acids without additional ionizable groups on their side chains, the isoelectric point occurs in a solution that is close to neutral pH (the pKa of the amine is greater than 7 while the pK+ of the carboxyl group is less than 7). For those with ionizable side chains, the isoelectric point is far from neutrality.