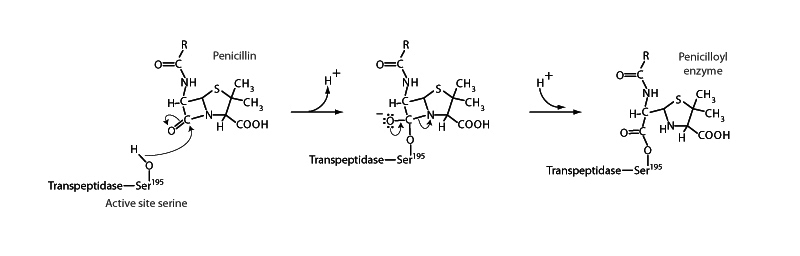
Penicillin belongs to the class of β-lactam antibiotics, possessing a β-lactam moeity in their molecular structure. A β-lactam is cyclic amide formed between a carboxyl group and an amine group two carbons away, making a four membered ring. Penicillin acts by inhibiting the synthesis of the peptidoglycan layer of bacterial cell walls by targeting transpeptidase, which catalyzes the final cross-linking step in peptidoglycan synthesis. The β-lactam moeity of penicillin irreversibly binds to a serine residue in the transpeptidase active site.
Unlike γ- or β-lactams, which form five- and six- membered rings, respectively, the four-membered ring of a β-lactam suffers under a great deal of angle strain. The lactam ring of penicillin is primed to break open, which is what occurs when the penicillin molecule encounters the transpeptidase active site. Through the acyl substitution pathway, the lactam carbon forms a covalent ester linkage with a reactive serine residue, breaking the amide, and the ring opens. Penicillin is now bound within the active site of the enzyme, an irreversible inhibitor of transpeptidase.