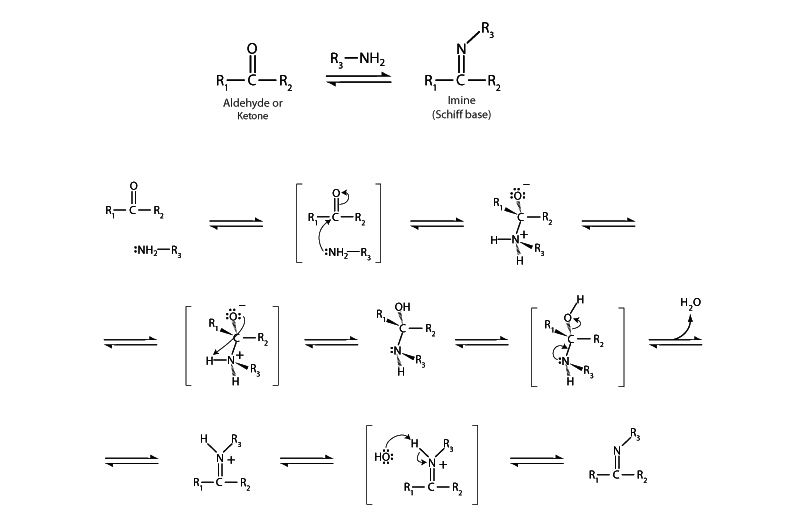
Imine formation involves the addition of a nucleophile to the carbonyl carbon of an aldehyde or ketone just like acetal formation, another important reaction in the category of nucleophilic additions to aldehydes and ketones. In acetal formation the nucleophile is an alcohol while in imine formation the nucleophile is a primary amine. The mechanism with imine formation is somewhat different concerning what happens after the addition. The overall process of imine formation produces a compound in which the C=O double bond has been replaced by a C=N double bond. This product is called an imine by organic chemists, but biochemists call this type of compound a Schiff base.
Comparing the mechanism of imine formation to acetal formation helps the understanding of both. In both reactions, after addition of the nucleophile, dehydration resolves the tetrahedral intermediate to a double-bonded, resonant cation. The oxonium
cation in acetal formation then becomes a target for an additional alcohol addition. With imine formation, however, the nitrogen still possesses a bond to hydrogen which serves as an electron source to resolve its electron deficiency with the simple loss of the proton, so there is no necessity for another nucleophilic attack. Because it resolves itself with an internal shift, imine formation is like acetal formation except that it stops a step earlier.