Interdisciplinary Note (2 of 25)
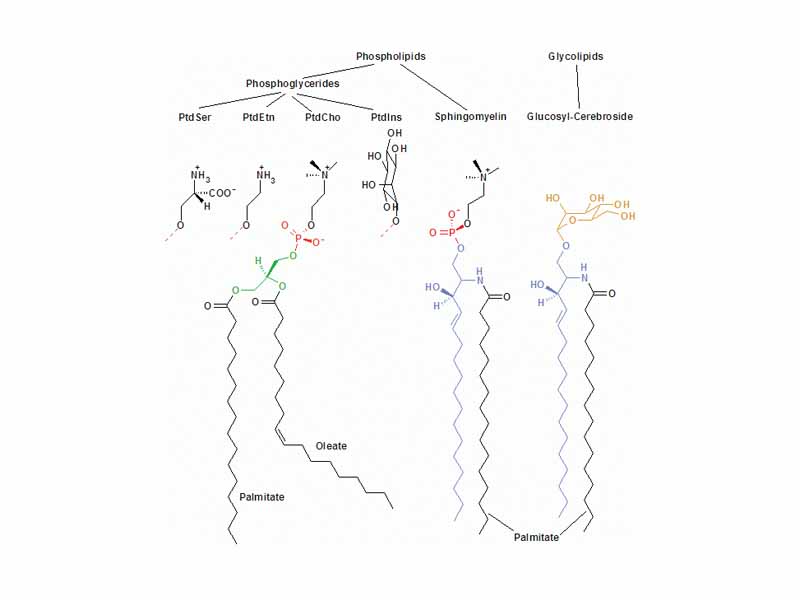
The three major membrane lipids are phospholipids, glycolipids, and cholesterol. These types are all amphipathic, containing both hydrophilic and hydrophobic moeities. Phospholipids and glycolipids spontaneously form bimolecular sheets in aqueous solution. The formation of such a bilayer maximizes the access of polar molecules for other polar molecules, water for itself and water for the polar heads of the lipid molecules. London dispersion forces enable close packing of the hydrocarbon tails. In thermodynamic terms, the bilayer is a favored state of the system because it represents a minimum of electrostatic potential energy, internal energy, enthalpy, and free energy. A water molecule is released from the interior of the bilayer because the charge densities represented by its poles are in a higher potential energy state in the midst of a nonpolar environment. These thermodynamic factors minimize the exposure of hydrocarbon tales to water and enable lipid bilayers to form extensive sheets that eventually close in on themselves to prevent there being edges to the sheet. In other words, an aqueous emulsion of phospholipid tends spontaneously toward segregation into compartmentalized spaced separated by the semipermeable bilayer.