Interdisciplinary Note (25 of 36)
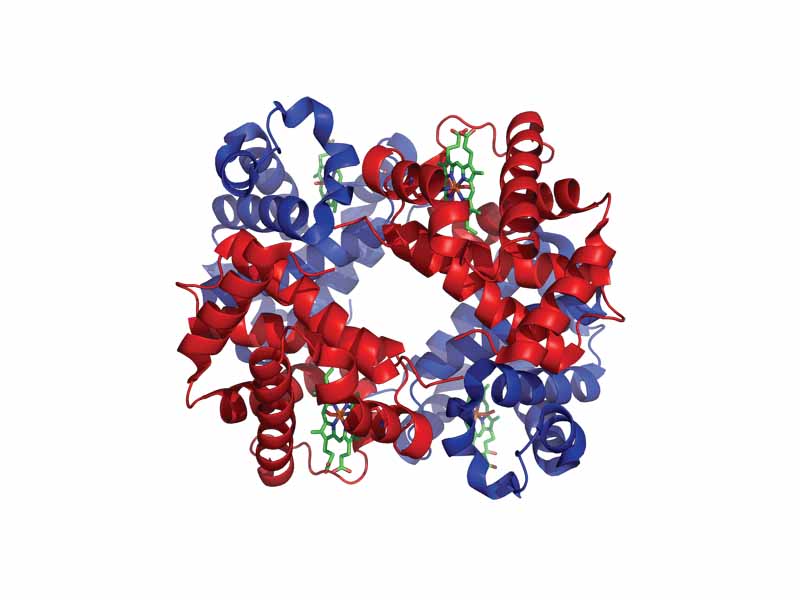
Favorite proteins of the test-writers, myoglobin and hemoglobin are oxygen carrying proteins in vertebrate organisms. Hemoglobin is the oxygen carrier in the blood. Hemoglobin also plays an important roll in carbon dioxide and hydrogen ion transport. Myoglobin helps to maintain a reserve supply of oxygen in the muscles. Hemoglobin is an allosteric protein in which the binding of O2 is controlled by H+, phosphate, and CO2 levels. Hemoglobin consists of four polypeptide chains connected by noncovalent forces (van der Waals, dipole-dipole, hydrogen bonding, and salt bridges). Each chain contains a nested heme group, in which iron forms a coordination complex in the center of the protoporphyrin ring, forming four bonds, leaving two additional coordination positions (one for a histidine side chain and one for oxygen). Hemoglobin is an allosteric protein, the binding of a molecule to one site on the protein leads to conformational changes that affect a distant site on the protein. O2 binding is cooperative (the first binding enhances additional binding by the other heme groups nested in the other polypeptide chains. Additionally, the binding of H+ or CO2 allosterically alters the binding of O2. The cooperative binding of oxygen by hemoglobin enables it to deliver much more oxygen within the capillaries than if the binding sites were independent. Additionally, higher levels of CO2 and H+ in the capillaries facilitates the release of oxygen from oxyhemoglobin (Bohr effect). (Additionally, 2,3 biphosphoglycerate (BPG) plays an important roll in modulating the oxygen affinity of hemoglobin, the difference in affinity for BPG, for example, accounting for the greater affinity of fetal hemoglobin F for oxygen).