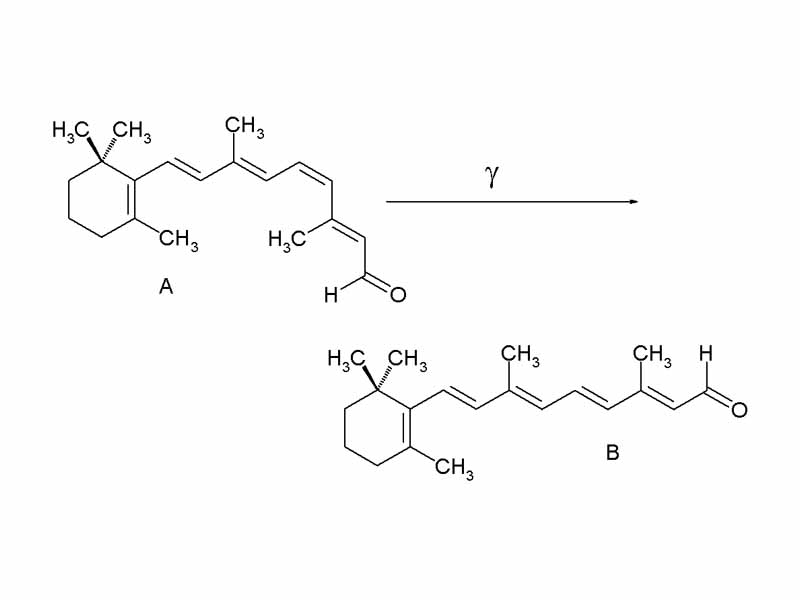
Interdisciplinary Note (4 of 36)
Visible light is 'visible' because the energy of photons in this range of the spectrum corresponds to the excitation energy of pigment molecules. In humans this is the vitamin A-based retinaldehyde chromophores bound to the protein opsin in the human visual system. Visible light has just the right amount of energy to elevate electrons between molecular orbitals and absorb light energy. The extended conjugated systems within pigments are essential in the absorbance qualities of these molecules. Lower energy light, such as infrared, does not elevate electrons between molecular orbitals in most chemical substances. Higher energy light, ultraviolet, can be destructive, often catalyzing chemical reactions.