Interdisciplinary Note (8 of 11)
Infrared radiation is the portion of the electromagnetic spectrum with wavelength longer than visible light but shorter than microwaves and other radio waves. The wavelengths of the portion of the IR spectrum used in IR spectroscopy are from 2.5 X 10-6m to 16 X 10-6m. (A unit commonly employed is the reciprocal centimeter (cm-1 or wavenumber) to refer to this range of wavelength. This way, the scale increases with energy 4000 cm-1 is the reciprocal of 2.5 X 10-6m and 625 cm-1 is the reciprocal of 16 X 10-6m.
IR radiation is readily absorbed by molecular substances because photon energy in this region corresponds to the difference between vibrational energy states corresponding to stretching and bending of atoms on chemical bonds.
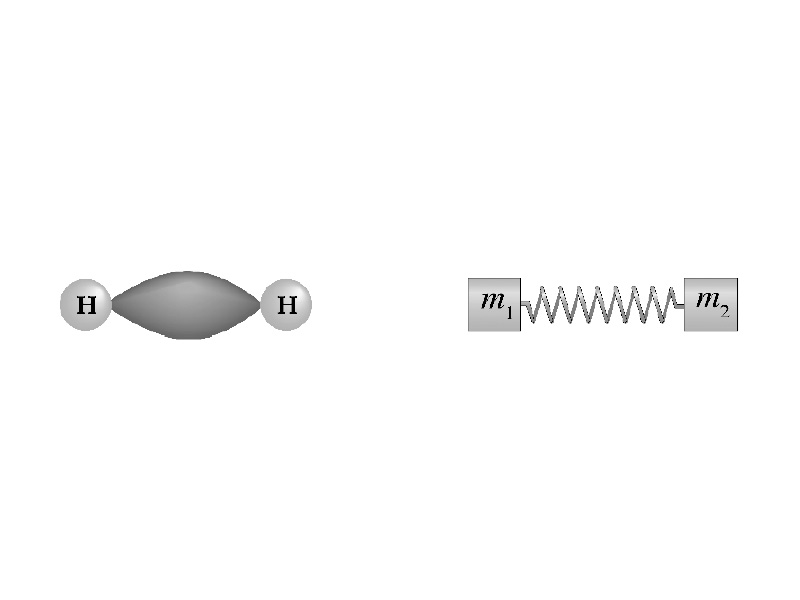
Although the force gradient is different, the mass-spring system can give useful insight into the vibrational modes along chemical bonds that give rise to infrared absorbances detected by IR spectroscopy. The relationships between electrostatic force, particle mass, and frequency are similar to the relationships between spring constant, object mass, and frequency of spring oscillation (although the force of attraction is very different). The main point is that larger masses and weaker forces give lower frequency. In general, this means that a singly bonded large atom absorbs at lower frequency than a double bonded small atom.