Interdisciplinary Note (27 of 36)
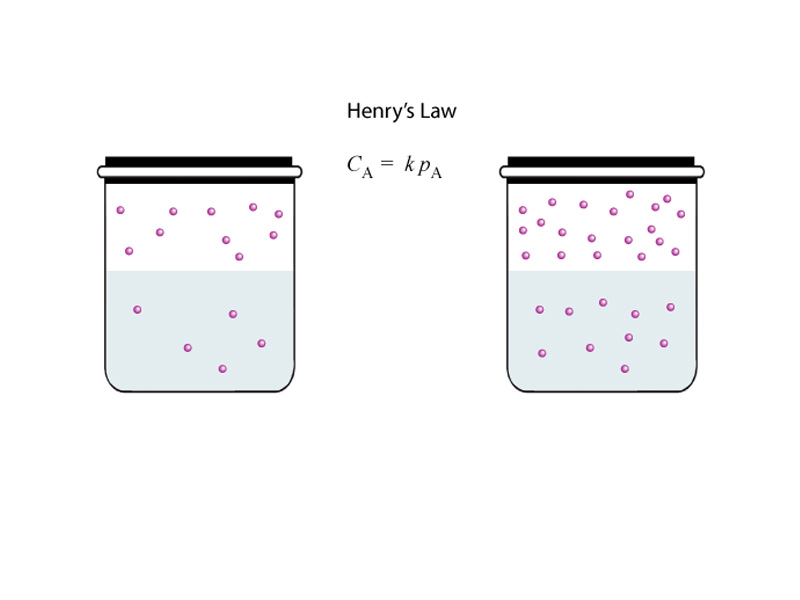
Human life occurs beneath an atmosphere 200 miles high, creating significant atmospheric pressure. The partial pressure of a component gas is proportional to its mole fraction. Oxygen represents 21% of air by molar concentration, and so exerts 21% of the air pressure (0.21 x 760mmHg = 160 mmHg). Although some gases are more soluble than others, for a particular gas, the amount which will dissolve in a liquid solvent increases in proportion to this partial pressure of the gas, an important physical determinant of the quantity ultimately delivered to the tissues. The concentration of a dissolved gas in a liquid is also a partial pressure exerting across membranes between fluid compartments and will determine the diffusion between fluid compartments. In other words, the reasoning is similar whether the gas is a component of a gaseous mixture or dissolved within a liquid solvent. Gas exchange involves diffusion into and out of the alveoli, intercellular fluid, capillaries, and erythrocytes. In the lungs oxygen diffuses from the alveolar air into the red blood cells, with carbon dioxide flow in the opposite direction. In the tissues, these flows are reversed, with oxygen flowing from the blood to the tissues and carbon dioxide from the tissues to the blood.
In fact, though, the solubility of gases in liquids is not high enough by itself to support a high metabolic rate. Hemoglobin and carbonic anhydrase both function to overcome the low solubility of gases (O2 and CO) in liquids.
Breathing is controlled by the medulla of the brain in response to chemoreceptors detecting changes in H+ and CO2 and O2, as well as baroreceptors detecting changes in blood pressure, and mechanoreceptors in the muscles.