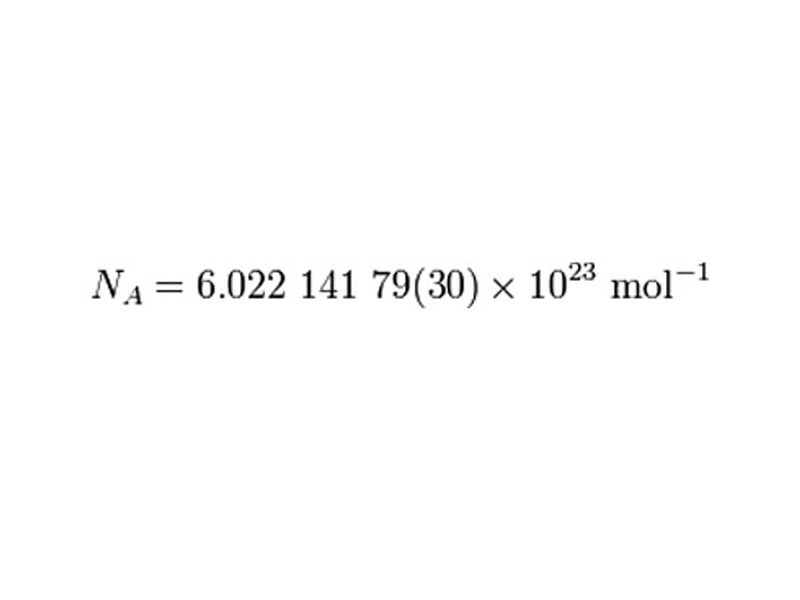There are real substances and there are the formulas employed in chemistry to represent these substances. Single, isolated, non-bonded atoms are rare in nature, with the exception of the noble gases. Most substances, even in pure elemental form, exist as discrete molecules or in crystalline form. Compounds contain two or more elements in chemical combination in fixed proportions. Each type of chemical formula, whether molecular, empirical or structural, provides a limited picture of the nature of the substances they describe. While the molecular formula does reflect the chemical composition of the compound, the structural formula will contain more information about the geometry, or stereochemistry. The empirical formula shows only the simplest ratio in which atoms occur in the substance.
In the laboratory, you typically measure the amount of a substance with a scale telling you how many grams of matter is present in your sample. But at the fundamental level, chemical interactions do not happen between 'grams', chemistry happens with particles. If you want to intelligently discuss your sample in the chemical context, it usually much more useful to talk about the amount of substance as a number of particles. But the number of particles is huge. It really is inconvenient to use numbers with twenty three zeros. For this reason, the concept of the mole was invented. The concept of a mole is extremely useful in that it provides a convenient way to discuss numbers of atoms or molecules that are relevant to laboratory scale samples. Many students get a bit confused by the mole. It helps to think of a 'a mole' as exactly the same kind of concept as 'a dozen', but instead of '12', the number of things in a mole is 6.02 X 1023.
The concept of the formula weight for a particular substance allows you to translate the grams you can measure in the laboratory into the moles you can use in chemical reasoning. The formula weight of a substance contains Avogadro's number of particles. One can measure out 2g of hydrogen gas or 32g oxygen gas, both representing one mole of gaseous substance, the same number of molecules.
In the laboratory, you typically measure the amount of a substance with a scale telling you how many grams of matter is present in your sample. But at the fundamental level, chemical interactions do not happen between 'grams', chemistry happens with particles. If you want to intelligently discuss your sample in the chemical context, it usually much more useful to talk about the amount of substance as a number of particles. But the number of particles is huge. It really is inconvenient to use numbers with twenty three zeros. For this reason, the concept of the mole was invented. The concept of a mole is extremely useful in that it provides a convenient way to discuss numbers of atoms or molecules that are relevant to laboratory scale samples. Many students get a bit confused by the mole. It helps to think of a 'a mole' as exactly the same kind of concept as 'a dozen', but instead of '12', the number of things in a mole is 6.02 X 1023.
The concept of the formula weight for a particular substance allows you to translate the grams you can measure in the laboratory into the moles you can use in chemical reasoning. The formula weight of a substance contains Avogadro's number of particles. One can measure out 2g of hydrogen gas or 32g oxygen gas, both representing one mole of gaseous substance, the same number of molecules.