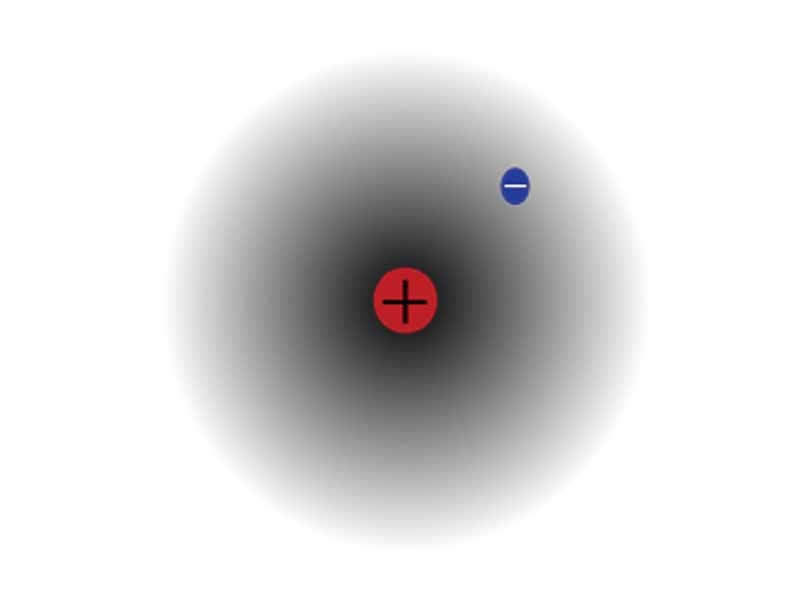
A consistent theme in this MCAT course is how helpful classical electrodynamics is to understanding chemistry. While quantum electrodynamics is needed for a complete picture, plain classical electrostatics provides an essential heuristic framework for understanding the periodic properties such as atomic radius, ionization energy, and electron affinity. Imagine mechanical work against electrostatic force to visualize the energy transformations of the atom.
Don't forget that atomic energy levels are quantized! Without forgetting that, you can still apply the simple, classical ideas that govern charge interaction to see how it is that atomic radius decreases as you move rightward on the table, for example. Imagine the stronger, more positive nucleus, pulling the electrons tighter with a stronger electrostatic force due to greater effective nuclear charge. Ionization energy increases moving rightward. Imagine how it takes more work to pull an electron in a given shell away from a nucleus with more protons. Put the ideas in motion in imaginative visualization to build your intuition.
The classical picture can help you as long as you know its limitations. In summary, it is often helpful to think about the periodic properties as if they were describing a simple electrostatic system as long as you don't forget that quantum electrodynamics provides a fuller picture.
Don't forget that atomic energy levels are quantized! Without forgetting that, you can still apply the simple, classical ideas that govern charge interaction to see how it is that atomic radius decreases as you move rightward on the table, for example. Imagine the stronger, more positive nucleus, pulling the electrons tighter with a stronger electrostatic force due to greater effective nuclear charge. Ionization energy increases moving rightward. Imagine how it takes more work to pull an electron in a given shell away from a nucleus with more protons. Put the ideas in motion in imaginative visualization to build your intuition.
The classical picture can help you as long as you know its limitations. In summary, it is often helpful to think about the periodic properties as if they were describing a simple electrostatic system as long as you don't forget that quantum electrodynamics provides a fuller picture.