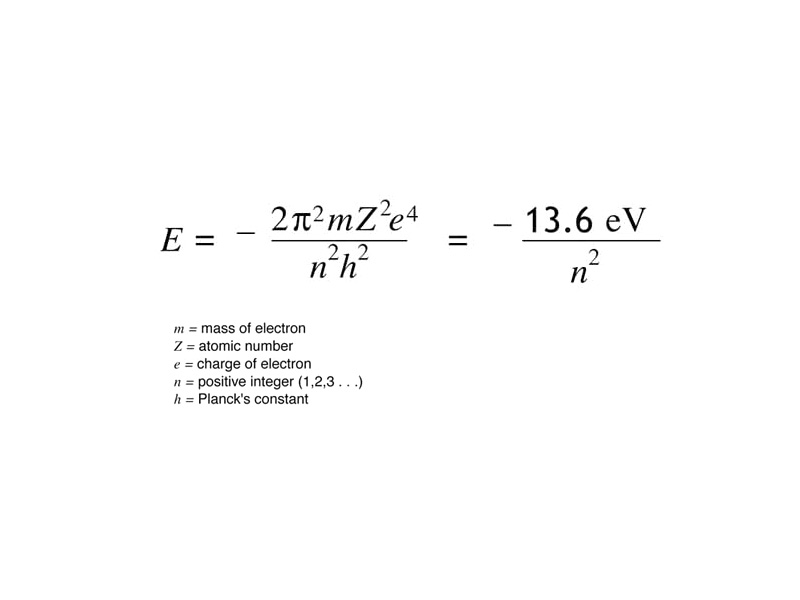
Our MCAT course is now beginning its transition from the fundamentals of classical physics, including electrostatics, to the beginnings of chemistry in atomic theory. As we make this transition from basic physics to chemistry, reflect carefully on preserving the insights that the classical model gives you while keeping in mind that the systems under discussion are quantum electrodynamical. How is the Bohr energy description of the atom similar to the classical description of the potential energy between oppositely charged particles? For one, the Bohr energy does include the contribution of kinetic energy, but that's an advanced footnote for our purposes. Both models present a picture in which oppositely charged particles have a potential energy of zero at infinite separation, with negative values reflecting decreasing potential energy as the electron and nucleus come nearer together.

How is the Bohr model different than the classical model described in the formula above? If the nucleus and electron of a hydrogen atom comprised a 'classical' rather than a quantized electrodynamic system, the potential energy function of an electron would be a continuous, and the emission spectrum, which derives from internal energy changes, would be continuous like a rainbow. In the Bohr model, however, the electron energy states are quantized, and the observed emission spectrum is a line spectrum corresponding to transitions between quantized energy levels.

How is the Bohr model different than the classical model described in the formula above? If the nucleus and electron of a hydrogen atom comprised a 'classical' rather than a quantized electrodynamic system, the potential energy function of an electron would be a continuous, and the emission spectrum, which derives from internal energy changes, would be continuous like a rainbow. In the Bohr model, however, the electron energy states are quantized, and the observed emission spectrum is a line spectrum corresponding to transitions between quantized energy levels.